Abstract
Background
Doripenem is the most recently introduced antimicrobial agent of the carbapenem class. It is a valuable therapeutic option in the context of increasing antimicrobial resistance to imipenem and meropenem among gram-negative bacilli (GNB) clinical isolates. However, clinicians are usually reluctant to prescribe doripenem, because susceptibility to doripenem is not automatically reported by most clinical laboratories and the in vitro activity of doripenem against clinically significant GNB isolates remains uncertain.
Materials and Methods
We investigated the in vitro antibacterial activity of doripenem in GNB blood isolates in a tertiary care center. Over a period of 10 months, 212 adult bacteremia cases were treated at the study hospital. Doripenem susceptibility testing was performed for the 212 blood isolates by the disk diffusion method, and clinical data were collected.
Results
Among the blood isolates, the rate of doripenem resistance (7.5%) was lower than that of imipenem (12.9%) or other anti-GNB antimicrobial agents, except amikacin (2.1%). Almost all imipenem-susceptible GNB blood isolates (181/182, 99.5%) were susceptible to doripenem. Whereas doripenem resistance was rarely observed in Enterobacteriaceae (2/181, 1.1%), it was frequently observed in patients with non-fermentatative GNB (12/27, 44.4%), hospital-acquired infections (7/27, 25.9%), and pneumonia (11/49, 22.4%).
Conclusion
Doripenem exhibited more potent in vitro activity against GNB blood isolates than other anti-GNB antimicrobial agents in a tertiary care center where it was infrequently prescribed compared with other carbapenems. However, its clinical utility may be limited due to the increasing number of carbapenem-resistant non-fermentative GNB infections.
Carbapenems (imipenem and meropenem) have been effective in the treatment of serious infections caused by antibiotic-resistant bacteria, especially multidrug-resistant gram-negative bacilli (MDR-GNB), during the last two or three decades [12]. However, the number of serious MDR-GNB infections with decreased antimicrobial susceptibility to carbapenems has recently increased [34]. In this context, doripenem, the latest introduced agent of the carbapenem class, may be a useful alternative treatment option as it shows potent in vitro antimicrobial activity against MDR-GNB and clinical effectiveness comparable with other carbapenems [56789]. However, clinicians are usually reluctant to prescribe doripenem, because data regarding doripenem susceptibility are rarely available from widely used automated susceptibility testing panels [10] and the in vitro activity of doripenem against clinically significant isolates such as blood isolates has not been reported separately in the context of recent increases in carbapenem resistance, except for a few studies [1112]. One included only blood isolates from bacteremic urinary tract infections, and the other did not include distinct clinical information on bacteremia cases. Therefore, we investigated the doripenem susceptibility of GNB blood isolates from adult patients and correlated it with their clinical data, and compared the in vitro antimicrobial activity of doripenem to those of imipenem and other anti-GNB antimicrobial agents in a tertiary care center where doripenem was infrequently prescribed and carbapenem resistance was increasing.
This study was performed at Chung-Ang University Hospital, an 850-bed tertiary care-affiliated hospital in Seoul, Republic of Korea. Between March and December 2012, we identified adult (≥20 years of age) patients whose blood cultures yielded GNB using the computerized database of the study hospital. We reviewed the electronic medical charts of the study patients and collected data on patient demographics, acquisition of infection, underlying diseases/conditions, initial severity of infection, antimicrobial therapy, and mortality.
Bacteremia was classified as community-acquired, healthcare-associated, or hospital-acquired, as described elsewhere [13]. Systemic inflammatory response syndrome (SIRS) criteria were defined as described elsewhere [14]. A site of infection was identified if it was clinically or microbiologically documented. If multiple sites of infection were found in one case, each site was separately recorded. The amount of carbapenem utilization during 2012 at the study hospital was quantified using the total number of defined daily dose (DDD) of the prescribed carbapenems. Carbapenem DDDs were as follows: meropenem (2.0 g), imipenem (2.0 g), and doripenem (1.5 g).
Identification and susceptibility testing of clinical isolates were performed using a Vitek II system (bioMérieux, Hazelwood, MO, USA). During the study period, meropenem susceptibility was not reported for 177 cases (83.5% of 212) due to the process of changing antimicrobial susceptibility panels in the study hospital. Thus, we excluded the reported meropenem susceptibilities of the remaining 35 isolates from the study analysis. Doripenem susceptibility testing was performed separately using the disk diffusion method in accordance with the recommendations of the Clinical and Laboratory Standards Institute (CLSI), because doripenem was not included in the susceptibility panels of the system. Susceptibility was determined based on the diameters of inhibition zones as follows: for Enterobacteriaceae, ≥23 mm (susceptible), 20-22 mm (intermediate resistant), ≤19 mm (resistant); for Pseudomonas aeruginosa, ≥19 mm (susceptible), 16-18 mm (intermediate resistant), ≤15 mm (resistant); for Acinetobacter spp., ≥18 mm (susceptible), 15-17 mm (intermediate resistant), ≤14 mm (resistant) [15]. Isolates with the result of either "intermediate resistant" or "resistant" were regarded as "resistant". This study was approved by the Institutional Review Board of the Chung-Ang University Hospital [IRB number, C2012019(714)].
In the study hospital, the doripenem DDD was 7.3% that of other carbapenems (499 of 6,871 DDDs; 5,418 DDDs for meropenem and 1,453 DDDs for imipenem) in 2012. A total of 212 episodes of GNB bacteremia occurred during the study period. Of these, 8 were polymicrobial cases of GNB and other bacteria, especially gram-positive cocci. Clinical characteristics of the cases are presented in Table 1. More than half of the cases were healthcare-associated or hospital-acquired infections (119, 56.1%). Diabetes mellitus was the most common underlying disease (60, 28.3%), followed by solid tumor (55, 25.9%), neurologic disorders (49, 23.1%), and heart failure (24, 11.3%). Escherichia coli and Klebsiella pneumoniae were most commonly detected (151, 71.2%). Other Enterobacteriaceae and non-fermentative GNB were each found in 30 cases (14.2%). The rate of doripenem resistance (7.5%) was lower than that of imipenem (12.9%) and those of the other anti-GNB antimicrobial agents except amikacin (2.1%). More than a quarter of cases (60, 28.3%) initially presented with severe sepsis or septic shock. The urinary tract was the most common site of infection (96, 45.3%), followed by the lungs (49, 23.1%), central venous catheter (36, 17.0%), abdomen (19, 9.0%), and skin/soft tissue (14, 6.6%).
Doripenem and imipenem susceptibilities are compared in Table 2. Almost all imipenem-susceptible isolates were susceptible to doripenem (181/182, 99.5%). One half of the imipenem-resistant isolates (13/26, 50.0%) were also susceptible to doripenem. Doripenem resistance was observed only rarely in the Enterobacteriaceae (2/181, 1.1%). However, for non-fermentative GNB, doripenem resistance was observed in the majority of imipenem-resistant isolates (84.6%, 11/13) and even in a case of imipenem-susceptible A. baumannii bacteremia.
Table 3 presents the rates of antimicrobial resistances of GNB bacteremia isolates according to type of pathogen, site of infection, and type of acquisition. The rate of doripenem resistance was lower than those of the other anti-GNB antimicrobial agents, including imipenem. However, the rate of doripenem resistance was high in cases of Pseudomonas aeruginosa bacteremia (40.0%) and Acintobacter spp. bacteremia (47.1%). It was also high in cases of pneumonia and hospital-acquired infection (22.4% and 25.9%, respectively). Among hospital-acquired pneumonia cases, the rate of doripenem resistance was 33.3% (4 of 12).
Imipenem-susceptible GNB blood isolates were also susceptible to doripenem in almost all cases. Doripenem was more active in vitro against GNB blood isolates than other anti-GNB antimicrobial agents, including imipenem. However, in non-fermentative GNB blood isolates and in clinical infections frequently associated with these organisms, especially hospital-acquired pneumonia, the in vitro activity of doripenem was limited like that of imipenem.
Imipenem-susceptible GNB blood isolates were also susceptible to doripenem in almost all cases. Although doripenem susceptibility is not routinely reported due to the absence of doripenem in automated susceptibility testing panels, our data suggest that if a GNB blood isolate is susceptible to imipenem, doripenem may be utilized effectively against it. The majority of previous studies also support this suggestion, finding that the in vitro activity of doripenem is similar to those of imipenem or meropenem, or sometimes superior to that of imipenem [56789]. A recent study specifically noted that meropenem and imipenem are reliable surrogate markers of doripenem susceptibility according to the surrogate predictive value based on the 2012 CLSI and FDA breakpoints [10]. However, the majority of previous studies included clinical isolates collected before 2010 [5678910]. At that time, doripenem had just been introduced and was not yet widely utilized in clinical practice. This study is not different from previous studies in some ways - doripenem was prescribed only at a rate of 7.4% compared to other carbapenems in this study. Thus, in a clinical setting where doripenem is more frequently prescribed than other carbapenems, doripenem susceptibility may not be predicted based on the susceptibilities of other carbapenems.
Our data showed that doripenem had potent in vitro activity against GNB blood isolates compared with other antimicrobial agents, including imipenem. In vitro activity of doripenem is reported to be comparable to those of other carbapenems [56789]. In addition, regarding clinical outcomes of complicated urinary tract infection, complicated intra-abdominal infection, nosocomial pneumonia, and bacteremia cases, doripenem shows similar effectiveness compared with other carbapenems [161718]. These data suggest that doripenem is a valuable antimicrobial treatment option against serious GNB infections. Additionally, considering that the majority of imipenem-resistant Enterobacteriaceae (11/13, 84.6%) were susceptible to doripenem in this study, we suggest that if antimicrobial treatment options are limited against imipenem-resistant Enterobacteriaceae blood isolates, antimicrobial susceptibility tests for doripenem would be helpful to detect additional therapeutic options.
We frequently observed antimicrobial resistance to doripenem among non-fermentative GNB blood isolates in this study. In a previous study that investigated the in vitro activity of doripenem in antibiotic-resistant clinical isolates collected from 2001-2002, doripenem was more potent than imipenem or meropenem even against non-fermentative GNB [19]. However, more recent studies of blood GNB isolates reported that doripenem and other carbapenems had similar in vitro activity against non-fermentative GNB, similar to the results of our study [1112]. Thus, doripenem should not be considered a treatment option against imipenem-or meropenem-resistant non-fermentative GNB blood isolates, or against hospital-acquired infections or pneumonia in which these GNB isolates are suggested as likely pathogens.
This study has a few important limitations. First, clinical data were retrospectively collected. It might affect the accuracy of the classification of hospital-acquired, healthcare-associated, and community-acquired infection. Second, meropenem susceptibilities could be evaluated only in 35 isolates. Thus, these were not included in the study analysis.
In conclusion, doripenem had more potent in vitro activity against GNB blood isolates than other anti-GNB antimicrobial agents, including imipenem, in a clinical setting where doripenem was infrequently utilized. However, its usefulness in clinical practice may be limited by the increasing number of carbapenem-resistant non-fermentative GNB.
Figures and Tables
Table 1
Clinical characteristics of gram-negative bacilli (GNB) bacteremia cases in a tertiary care center and rates of antimicrobial resistance of the GNB blood isolates

Table 2
Doripenem and imipenem susceptibilities of 208 GNB blood isolates (after the exclusion of Aeromonas hydrophila and Stenotrophomonas maltophilia)
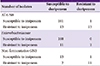
Table 3
Antimicrobial resistances of anti-gram negative bacilli (GNB) antimicrobial agents according to type of pathogen, site of infection, and type of acquisition among GNB blood isolates in a tertiary care center

References
1. Baldwin CM, Lyseng-Williamson KA, Keam SJ. Meropenem: a review of its use in the treatment of serious bacterial infections. Drugs. 2008; 68:803–838.
2. Rodloff AC, Goldstein EJ, Torres A. Two decades of imipenem therapy. J Antimicrob Chemother. 2006; 58:916–929.

3. Pogue JM, Mann T, Barber KE, Kaye KS. Carbapenem-resistant Acinetobacter baumannii: epidemiology, surveillance, and management. Expert Rev Anti Infect Ther. 2013; 11:383–393.

4. Kanj SS, Kanafani ZA. Current concepts in antimicrobial therapy against resistant gram-negative organisms: extended-spectrum beta-lactamase-producing Enterobacteriaceae, carbapenem-resistant Enterobacteriaceae, and multidrug-resistant Pseudomonas aeruginosa. Mayo Clin Proc. 2011; 86:250–259.

5. Jones RN, Huynh HK, Biedenbach DJ, Fritsche TR, Sader HS. Doripenem (S-4661), a novel carbapenem: comparative activity against contemporary pathogens including bactericidal action and preliminary in vitro methods evaluations. J Antimicrob Chemother. 2004; 54:144–154.

6. Mushtaq S, Ge Y, Livermore DM. Doripenem versus Pseudomonas aeruginosa in vitro: activity against characterized isolates, mutants and transconjugants and resistance selection potential. Antimicrob Agents Chemother. 2004; 48:3086–3092.

7. Fritsche TR, Stilwell MG, Jones RN. Antimicrobial activity of doripenem (S-4661): a global surveillance report (2003). Clin Microbiol Infect. 2005; 11:974–984.

8. Castanheira M, Jones RN, Livermore DM. Antimicrobial activities of doripenem and other carbapenems against Pseudomonas aeruginosa, other nonfermentative bacilli, and Aeromonas spp. Diagn Microbiol Infect Dis. 2009; 63:426–433.

9. Livermore DM. Doripenem: antimicrobial profile and clinical potential. Diagn Microbiol Infect Dis. 2009; 63:455–458.

10. Hagihara M, Kuti JL, Nicolau DP. Predicting doripenem susceptibility based on meropenem and imipenem interpretation for Pseudomonas aeruginosa. Diagn Microbiol Infect Dis. 2012; 72:258–262.

11. Lee H, Ko KS, Song JH, Peck KR. Antimicrobial activity of doripenem and other carbapenems against gram-negative pathogens from Korea. Microb Drug Resist. 2011; 17:37–45.

12. Dong SX, Wang JT, Chang SC. Activities of doripenem against nosocomial bacteremic drug-resistant Gram-negative bacteria in a medical center in Taiwan. J Microbiol Immunol Infect. 2012; 45:459–464.

13. Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, Briggs JP, Lamm W, Clark C, MacFarquhar J, Walton AL, Reller LB, Sexton DJ. Health care-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002; 137:791–797.

14. Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992; 101:1644–1655.

15. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: 24th informational supplement. Wayne, PA: CLSI;2014. Document M100-S24.
16. Rice DA, Kaniga K, Lee M, Redman R. Activity of doripenem versus comparators in subjects with baseline bacteremia in six pooled phase 3 clinical trials. Int J Antimicrob Agents. 2013; 41:388–392.

17. Mustafa M, Chan WM, Lee C, Harijanto E, Loo CM, Van Kinh N, Anh ND, Garcia J. A PROspective study on the Usage patterns of Doripenem in the Asia-Pacific region (PROUD study). Int J Antimicrob Agents. 2014; 43:353–360.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download