Introduction
Over recent years, the increasing prevalence of antimicrobial-resistant bacteria has become a serious problem worldwide. Bacterial surveillance previously conducted in Korea indicated a high prevalence of methicillin-resistant Staphylococcus aureus (MRSA), penicillin G-resistant Streptococcus pneumoniae, vancomycin-resistant Enterococcus faecium (VR-EFM), and extended cephalosporin-resistant Enterobacteriaceae. The recent increase in multidrug-resistant (MDR) Acinetobacter spp. and Pseudomonas aeruginosa, and carbapenem-resistant Klebsiella pneumoniae is of great concern in many countries. The prevalence of resistant bacteria varies substantially from country to country, and even from hospital to hospital, as it is influenced significantly by antimicrobial use and the degree of success of efforts to control the spread of resistant bacteria.
Surveillance of antimicrobial resistance provides essential data that increases our understanding of the epidemiology of antimicrobial resistance and aids in optimizing empirical management regimens, driving pharmaceutical companies to develop new antimicrobial agents, and adopting better measures for controlling antimicrobial resistance. Since 1997, the Korean Nationwide Surveillance of Antimicrobial Resistance (KONSAR) program has conducted passive surveillance via the analysis of data from tests conducted by participating laboratories [
1]. A study in 2009 showed a continued high prevalence of MRSA, third-generation cephalosporin-resistant
K. pneumoniae, and fluoroquinolone-resistant
Escherichia coli,
P. aeruginosa, and
Acinetobacter spp. There was a minor increase in the prevalence of imipenem-resistant
P. aeruginosa, but there was a dramatic increase in the prevalence of imipenem-resistant
Acinetobacter spp. MDR microorganisms were much more common among isolates from intensive care units (ICUs) than those from non-ICUs.
The aim of this study was to analyze the antimicrobial resistance patterns of clinically significant bacteria isolated in 2011 in Korea. Resistance rates were compared between isolates from ICUs and those from non-ICUs. Resistance patterns of isolates derived from secondary care hospitals and primary care clinics that did not have clinical microbiology laboratories were provided by two commercial laboratories. Resistance patterns of isolates derived from tertiary care hospitals were also analyzed. The prevalence of colistin resistance was also assessed, due to the increasing prevalence of MDR Acinetobacter spp. and P. aeruginosa.
Materials and Methods
Antimicrobial susceptibility test data generated in 2011 by KONSAR-participating laboratories were collected from 32 hospitals and two commercial laboratories, which processed specimens at the request of secondary care hospitals and primary care clinics. Data from hospitals with poor quality performance, such as those indicating susceptibility to intrinsically resistant antimicrobials, were excluded. Resistance patterns of clinically significant microorganisms isolated from ICU patients were compared to organisms isolated from non-ICU patients using data from 20 hospitals.
Questionnaire responses revealed that one laboratory tested the susceptibility of
E. coli (representing Gram-negative bacilli) and
S. aureus (representing gram-positive cocci) using Clinical and Laboratory Standards Institute (CLSI) disk diffusion [
2], while 25 laboratories used commercial broth microdilution. One laboratory used both methods. Cefotaxime and ceftazidime susceptibility was interpreted using previous CLSI [
2] breakpoints. For
S. pneumoniae, the oxacillin disk method with meningitis breakpoint was used to screen penicillin G-non-susceptible isolates [
3].
The majority of laboratories used WHONET software [
4] to analyze susceptibility data. Duplicate isolates were excluded from the analysis. As in the 2009 study [
5], resistance rates did not include intermediate susceptibility and mean resistance rates were calculated by averaging hospital resistance rates to avoid the influence of high numbers of isolates from large hospitals. Hospitals that tested fewer than 10 isolates of an organism were excluded from the analysis to avoid bias [
6,
7]. The statistical significance of differences between resistance rates was not determined in this surveillance as this has been a common feature of large-scale and continuous surveillance programs [
8,
9].
Among the hospital laboratories that participated in the study, 22 modified the measured susceptibility results of cefotaxime/ceftriaxone, ceftazidime, cefepime, and aztreonam if the
E. coli and
K. pneumoniae isolates tested were extended-spectrum β-lactamase (ESBL) producers [
2,
3]. We therefore compared unmodified resistance rates for these antibiotics from the remaining five hospital laboratories with data from the previous KONSAR studies in order to maintain data consistency.
Discussion
The prevalence of antibiotic-resistant bacteria varies significantly by time, country, and patient population. Antimicrobial resistance surveillance studies are important for monitoring the levels of endemic or emerging resistance. This KONSAR study report showed that the majority of hospital laboratories used commercial broth microdilution methods for E. coli (Gram-negative) and S. aureus (Gram positive) susceptibility testing.
In terms of laboratory analysis of susceptibility data, the CLSI [
10] recommends including only the first isolate in cases of multiple isolates from a single patient, in order to effectively guide clinicians in the empirical selection of appropriate antimicrobial agents. In this study, we excluded duplicate isolates. However, excluding duplicate isolates may have the effect of underestimating the resistance rates of some organisms to certain antimicrobial agents, especially when this analysis is conducted only once a year. To detect the development of resistance during antimicrobial therapy, the CLSI [
3] recommends susceptibility testing to third-generation cephalosporins in
Enterobacter,
Citrobacter, and
Serratia spp. isolates obtained three to four days after initial isolation.
P. aeruginosa should be tested for susceptibility to all antimicrobial agents; and staphylococci should be tested for susceptibility to fluoroquinolones.
Although the commercial laboratory isolates included bacterial strains originating from hospitals, we tried to separate and compare the hospital and commercial laboratory isolates in order to follow up trends in each MDR microorganisms to make comparisons with previous KONSAR studies. In 2011,
E. coli,
S. aureus, CNS,
K. pneumoniae, and
P. aeruginosa were the five most prevalent organisms isolated by hospitals (
Table 1). Compared with 2009 data, the relative prevalences of
K. pneumoniae and
P. aeruginosa changed. In 2009,
K. pneumoniae was the fifth most prevalent and
P. aeruginosa the fourth most prevalent bacterial species isolated. Among the commercial laboratory isolates,
E. coli,
P. aeruginosa,
S. aureus,
K. pneumoniae and CNS (in order of decreasing prevalence) were the most commonly isolated.
E. faecalis was the seventh most common in 2011 but fifth in 2009. Among ICU isolates,
S. aureus,
Acinetobacter spp., CNS,
P. aeruginosa, and
K. pneumoniae were the five most commonly isolated microorganisms. EARSS/EARS-Net data from 2011 (
www.ecdc.europa.eu) showed that
E. coli was the most prevalent species, accounting for 73% (344,700 of 471,596) of bacterial isolates, followed by
K. pneumoniae (16%, 74,985 isolates) and
P. aeruginosa (11%, 51,911 isolates). These data are similar to our results.
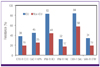
Antimicrobial pressure is much higher on ICUs than on general wards [
11,
12]. Analysis of ICU data from 20 hospitals in this study showed that extended-spectrum cephalosporin-resistant
E. coli and
K. pneumoniae, imipenem-resistant
Acinetobacter spp. and
P. aeruginosa, oxacillin/cefoxitin-resistant
S. aureus, and VR-EFM were more prevalent among ICU isolates than non-ICU isolates (
Fig. 1). A comparison of resistance in ICU isolates analyzed in this study with data from an international study showed that rates of MRSA and piperacillin-resistant
P. aeruginosa were similar to rates from regions outside the United States: 84% and 84.1% for MRSA, and 84% and 78% for piperacillin-resistant
P. aeruginosa [
13].
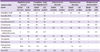
In Korea, hospital-associated MRSA is a serious problem. In this study, the prevalence of MRSA was 66% in both hospital- and commercial laboratory-tested isolates (
Table 2,
Fig. 2). Of the invasive
S. aureus isolates reported in the 2011 EARS-Net data, 54.6% of 1,307
S. aureus isolates from Portugal, 20.1% of 4,716 isolates from France, and 13.6% of 3,408 isolates from the United Kingdom were non-susceptible to oxacillin (
www.ecdc.europa.eu).
Vancomycin-resistant enterococci are also important Gram-positive nosocomial pathogens (
Table 2). In this study, vancomycin resistance was 1.2% for hospital-isolated
E. faecalis and 23% for hospital-isolated
E. faecium. In 2011, the resistance rates of vancomycin in Denmark, Finland, and France were found to be ≤ 1%, while the rates in Germany and Greece were 11% and 23%, respectively (
www.ecdc.europa.eu). The European report also showed that resistance rates varied by hospital, even within countries.
Commercial laboratory-tested
E. faecium isolates showed slightly higher resistance rates to teicoplanin and vancomycin than isolates tested in hospital laboratories (
Table 2,
Fig. 1). These data suggest that the prevalence of nosocomial VR-EFM infection was common in participating secondary care hospitals and primary care clinics.
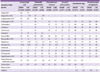
Interestingly, the resistance rates of
E. coli and
K. pneumoniae to cephalothin/cefazolin, piperacillin-tazobactam, amikacin, and ciprofloxacin/levofloxacin were lower among hospital isolates than among commercial laboratory isolates (
Table 3). In 2011, the CLSI [
3] lowered the resistance breakpoints of cefotaxime to ≥ 4 µg/mL, ceftazidime to ≥ 16 µg/mL, and aztreonam to ≥ 16 µg/mL. The CLSI also eliminated the requirement for ESBL testing of
E. coli,
K. pneumoniae spp., and
P. mirabilis. In this study, only five hospitals followed these guidelines, with the remaining hospitals testing for ESBL production. The rates of ESBL-positivity were slightly lower for hospital-isolated
E. coli and
K. pneumoniae than for commercial laboratory-tested isolates (
Table 3,
Fig. 2). The resistance rates of hospital-isolated
E. cloacae and
S. marcescens to cefotaxime, ceftazidime, and piperacillin-tazobactam were higher compared to those of isolates tested in commercial laboratories.
Currently, resistance to carbapenems is the most worrying type of antimicrobial resistance. Carbapenem resistance among
Acinetobacter spp. was higher than that among
P. aeruginosa (
Table 3,
Fig. 5,
Fig. 6). Resistance to imipenem was higher in hospital-tested
Acinetobacter spp. isolates than in commercial laboratory-tested isolates (
Table 3,
Fig. 2). In this study, 22% of hospital-isolated
P. aeruginosa were resistant to imipenem. This is much higher than the 5.5% observed in Denmark and 4.6% in the Netherlands, but lower than the 66.7% found in Romania (
www.ecdc.europa.eu). The imipenem resistance rate of
P. aeruginosa isolates tested in hospital laboratories was lower than for isolates tested in commercial laboratories.
Ampicillin resistance among hospital-isolated non-typhoidal
Salmonella spp. substantially increased from 20% in 2009 [
14] to 36% in 2011 (data not shown). Resistance rates differed significantly depending on the hospital, ranging from 3% to 83%. These data indicate the presence of local outbreaks in some settings. In this study, 53% of hospital isolates and 48% of commercial laboratory isolates of
H. influenzae were resistant to ampicillin, suggesting stability, since previous data were 58.5% in 2005 and 2006 [
15].
The prevalence of imipenem-resistant
P. aeruginosa decreased slightly between 2009 and 2011, while imipenem resistance in
Acinetobacter spp. increased (
Fig. 5 and
6). According to previous KONSAR studies, imipenem resistance rates among
P. aeruginosa isolates were 19% in 2005, 26% in 2009, and 22% in 2011. Among
Acinetobacter spp., resistance rates were 16% in 2005, 51% in 2009, and 64% in 2011. The drastic increase in the prevalence of imipenem-resistant
Acinetobacter spp. is mostly due to OXA-type carbapenemase production. Almost all carbapenem-resistant
A. baumannii carried
blaOXA-23-like or ISAba1-activated
blaOXA-51-like genes, while most non-
baumannii Acinetobacter carried metallo-β-lactamase genes [
16].
Colistin-resistant
A. baumannii isolates have previously been detected in Korea [
17]. While colistin-resistance rates in
Acinetobacter spp. isolated in Korea vary substantially between studies [
18], colistin susceptibility must be included in surveillance studies because colistin is the last resort for treatment of MDR
Acinetobacter spp. infections [
19]. In this study, the colistin resistance rate in hospital-isolated
Acinetobacter spp. was less than 2%, which was significantly lower than previous reports of 30.6% [
17] and 9.1% [
20].
The emergence of antibiotic resistance is inevitable and therefore efforts to decrease the impact of resistance and prolong the effectiveness of available agents are required. For this reason, surveillance of antimicrobial resistance must continue, in order to determine trends in emerging resistant organisms in Korea such as OXA-48 and OXA-232-producing Gram-negative bacilli [
21,
22].
In conclusion, this KONSAR study of data collected in 2011 showed that among bacteria isolated in hospital laboratories, the prevalence of ciprofloxacin/levofloxacin resistance and amikacin resistance in Acinetobacter spp. increased; imipenem resistance also increased substantially in this microorganism. The prevalence of carbapenem-resistant, amikacin-resistant, ceftazidime-resistant, and ciprofloxacin/levofloxacin-resistant P. aeruginosa, and ceftazidime-resistant, ciprofloxacin/levofloxacin-resistant, amikacin-resistant, and cefoxitin-resistant K. pneumoniae decreased compared with data from 2009. MDR organisms were much more common among isolates taken from ICUs.









 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download