Abstract
Mycotic aneurysms are uncommon, but are fatal without appropriate management. Previous reports have shown that anaerobes and gram-negative organisms are less common but more dangerous than other causative agents of mycotic aneurysm. We report the case of a 60-year-old man with poorly controlled diabetes mellitus and atherosclerosis in the aorta, and a 10-day of history of lower abdominal pain and fever. This man was diagnosed with an uncommon abdominal aorta mycotic aneurysm caused by Bacteroides thetaiotaomicron and Acinetobacter lwoffii. The aneurysm was successfully treated with antibiotics therapy and aorto-bi-external iliac artery bypass with debridement of the infected aortic wall. We present this case together with a review of the relevant literature.
The development of a mycotic aneurysm is an uncommon but fulminant process. It is potentially life threatening as it can lead to aortic aneurysm rupture, despite aggressive therapy [1, 2]. Staphylococcus aureus and Salmonella species are pathogens commonly associated with mycotic aneurysm development [3, 4, 5], but Bacteroides species have been reported rarely and Acinetobacter lwoffii has not previously been reported as causing mycotic aneurysm. We present a case of abdominal aortic mycotic aneurysm in a 60-year-old man who presented with fever and lower abdominal pain, caused by Bacteroides thetaiotaomicron and A. lwoffii.
A 60-year-old man was admitted to a hospital because of a 10-day history of lower abdominal pain and fever. His past medical history included diabetes mellitus, which was poorly controlled (HbA1c level of 11.2%). He had no history of recent infection, acupuncture, or intravenous injection.
On admission, he was febrile and complained of lower abdominal pain, but no abdominal tenderness or rebound tenderness was elicited. Other meaningful symptoms and signs suggesting metastatic complications (endophthalmitis, central nervous system infection, etc.) were not present. His initial vital signs included high fever (39.0℃), blood pressure of 140/80 mmHg, a heart rate of 92 beats/min, and a respiration rate of 20 breaths/min.
Laboratory investigations showed a white blood cell (WBC) count of 8,610/mm3 (neutrophils, 83.9%; lymphocytes, 7.3%; monocytes, 6.3%; basophils 0.3%), a hemoglobin level of 13.6 g/dL, and a platelet count of 122,000/mm3. The C-reactive protein (CRP) level was 16.56 mg/dL.
Enhanced computed tomography (CT) revealed a mycotic aneurysm originating from the abdominal aorta (Fig. 1), and no other infectious focus was observed on abdominal CT. A transthoracic echocardiogram did not reveal any evidence of infective endocarditis.
The patient was diagnosed with an abdominal aortic mycotic aneurysm. Because the patient had poorly controlled diabetes mellitus and mycotic aneurysm has a high mortality rate and can be caused by various organisms, strong broad-spectrum intravenous antibiotic therapy was immediately initiated (intravenous 1g q8hr of meropenem plus 200 mg q24hr of teicoplanin). The fever was controlled and the CRP level decreased during eight days of antibiotic therapy. However, even with intravenous antibiotics, he continued to complain of abdominal pain.
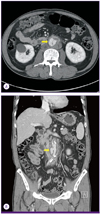
The patient's vital signs were stable without fever and laboratory-tested parameters did not worsen. However, we conducted a follow-up enhanced CT to assess the need for surgical management (8 hospital days). Enhanced CT showed an enlarged aneurysm in a state of impending rupture (Fig. 2). Surgery was immediately performed to address the infection. Massive atherosclerotic changes and plaque formation were observed in the intima of the abdominal aorta, and inflammatory change was noted from 1 cm below the renal artery bifurcation to the iliac artery bifurcation. The infected aortic wall was debrided and an aorto-bi-external iliac artery bypass was performed.
Later, two out of three anaerobic blood cultures were positive for unidentified bacilli. To identify the isolate, 16S rRNA was sequenced (Macrogen, Daejeon, Korea). In brief, DNA was extracted and PCR was performed using primers targeting the 16S rRNA region, followed by purification, the sequencing reaction, and analysis. The resulting sequence was compared with sequences stored in the GenBank (http://www.ncbi.nlm.nih.gov/genbank) and EzTaxon (http://www.eztaxon.org) databases, and one isolate was identified as B. thetaiotaomicron, with a percentage identity of 98.08%. Tissue biopsied from the wall of the aortic aneurysm was also positive for A. lwoffii, which was detected using standard microbiological methods. The following minimum inhibitory concentrations (MIC via VITEK II) were recorded: ampicillin/sulbactam 2; amikacin 2; ciprofloxacin 0.25; colistin 0.5; cefepime 1; imipenem 0.25; meropenem 0.25; ceftazidime 1; cefotaxime 8; and piperacillin/tazobactam 4. According to the microbiological results, the antibiotic therapy was changed from meropenem plus teicoplanin to ciprofloxacin plus metronidazole (intravenous 500 mg q12hr of ciprofloxacin and 500 mg q8hr of metronidazole).
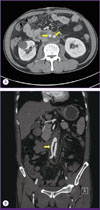
The day after surgery, the patient had no fever and laboratory data indicated an improvement. Enhanced CT performed on day 7 after surgery (Fig. 3) revealed no serious complications or re-infection. After 12 days of intravenous antibiotic therapy, the WBC count and CRP level had decreased to within the normal range. The patient recovered and was discharged. He remained in good condition and continued to take oral ciprofloxacin and metronidazole for 13 days after surgery. He is being followed-up at the outpatient clinic and is reported to be in good condition.
Mycotic aneurysm is a false aneurysm related to any intravascular infection or systemic infection. Mycotic aneurysms represent 0.7.3.3% of all aortic aneurysms [1, 3, 4, 6], and tend to occur at the major bifurcations of the aorta, most frequently the abdominal aorta and femoral artery [3]. This type of aneurysm has a high rate of rupture and subsequent mortality, with reported in-hospital mortality rates of 5.44% [4, 6].
Mycotic aneurysm can develop as a result of several processes: hematogenous spread to the intima or vasa vasorum, lymphatic spread, or direct extension from an adjacent infected focus [7, 8]. The risk factors for mycotic aneurysms are aortic trauma, congenital aortic anomalies, and conditions causing impaired immunity. Our patient had poorly controlled diabetes mellitus and atherosclerosis of the aorta, but no evidence of endocarditis or other previous infections. Diabetes mellitus results in impaired immunity, and disruption of the intima by atherosclerosis reduces resistance to infection [4]. In the present case, these risk factors were associated with the patient's condition.
Pathogens have been cultured from 46-74% of blood samples and 76% of tissue biopsy samples [5, 6]. The most frequent pathogens are S. aureus and Salmonella species [2, 3, 4], but other organisms (e.g., Bacteroides species) have been occasionally reported as causative pathogens [6, 9]. Jarrett et al. [10] reported that the incidence of aneurysm rupture and mortality is higher with gram-negative infections than with gram-positive infections (84% vs. 10% for rupture and 84% vs. 50% for morality, respectively).
In our case, three sets of blood cultures were collected and tissue from the infected aortic wall was cultured. After 3 days of incubation, gram-negative rods grew in two anaerobic blood culture bottles; subculture of the positive broth resulted in the growth of small colonies on a blood agar plate. However, the organism could not be identified using the Vitek 2 system (BioMérieux Inc., Marcy-l'Etoile, France), and was instead identified using 16S RNA sequencing as B. thetaiotaomicron. In addition, A. lwoffii was identified from the aortic tissue culture using the Vitek 2 Gram-Negative Identification card (BioMérieux Inc., France).
In Korea, there have been no reports of mycotic aneurysms caused by these pathogens. To the best of our knowledge, mycotic aneurysm caused by A. lwoffii has not been previously reported anywhere, and only one case of mycotic aneurysm caused by B. thetaiotaomicron has been previously described [6]. B. thetaiotaomicron is a gram-negative anaerobic microbe, constitutive of the normal flora of the vagina and intestinal tract. However, it is also the second most common infectious anaerobic gram-negative bacterium, although it is rarely isolated [11]. A. lwoffii is a gram-negative aerobic bacillus that is a component of the normal flora of the skin, oropharynx, and perineum. It is capable of causing gastritis [12].
As mentioned above, the blood and tissue cultures identified different organisms. Although we considered specimen contamination, this was unlikely for the following reasons. First, two out of three bottles were positive for Bacteroides species. Second, a blood culture positive for the B. fragilis group (including B. thetaiotaomicron) almost always, represents true infection [13]. Third, tissue specimens were obtained in an aseptic operating room, making contamination less likely. Anaerobic bacteria are difficult to isolate in cultures [14], and 7 days of antibiotic therapy was given before surgery. These factors may have suppressed growth of B. thetaiotaomicron. As both organisms were possible pathogens in this case, we prescribed antibiotics that covered both organisms.
Optimal management of mycotic aneurysms remains controversial, but many authors recommend a combination of antibiotic therapy and surgical excision. With medical treatment alone, the mortality rate is approximately 90%; combined medical and surgical treatment may reduce this rate to below 25% [1]. However, Muller et al. [4] reported that perioperative mortality can be approximately 63% in patients with aneurysmal rupture. Therefore, although there are no definitive guidelines for the use of antibiotics, many authors recommended that antibiotic therapy should continue for at least 6-12 weeks after surgery to control sepsis and achieve cardiovascular stability [2]. Currently, an attempt is being made to treat some patients with non-ruptured mycotic true aneurysms by endovascular methods. These methods do not completely remove the infected aorta or artery, so recurrent infection may lead to increased morbidity and mortality compared with primary surgical intervention. In a systematic review, Kan et al. [15] noted that, in 22 reports of mycotic aneurysms of the thoracic or abdominal aorta, a total of 48 patients were treated using the endovascular method. This review showed a 30-day survival rate of 89.6% and two-year survival rate of 82.2%. Despite long-term antibiotic therapy, recurrent infection occurred and re-operation was required. The authors recommended definitive surgical treatment for patients presenting with aneurysm rupture or fever.
To our knowledge, this is the first case in Korea of mycotic aneurysm caused by B. thetaiotaomicron and A. lwoffii. In the future, we hope definitive treatment guidelines will be established to improve the survival rate for patients with mycotic aneurysm.
Figures and Tables
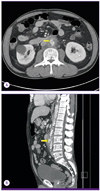 | Figure 1(A, B) A 2.1-cm (diameter) saccular aneurysm (arrows) with periaortic fluid collection was visible in the abdominal aorta at the L3 level. |
References
1. Lopes RJ, Almeida J, Dias PJ, Pinho P, Maciel MJ. Infectious thoracic aortitis: a literature review. Clin Cardiol. 2009; 32:488–490.

3. Malouf JF, Chandrasekaran K, Orszulak TA. Mycotic aneurysms of the thoracic aorta: a diagnostic challenge. Am J Med. 2003; 115:489–496.

4. Müller BT, Wegener OR, Grabitz K, Pillny M, Thomas L, Sandmann W. Mycotic aneurysms of the thoracic and abdominal aorta and iliac arteries: experience with anatomic and extra-anatomic repair in 33 cases. J Vasc Surg. 2001; 33:106–113.

5. Johnson JR, Ledgerwood AM, Lucas CE. Mycotic aneurysm. New concepts in therapy. Arch Surg. 1983; 118:577.
6. Maeda H, Umezawa H, Goshima M, et al. Primary infected abdominal aortic aneurysm: surgical procedures, early mortality rates, and a survey of the prevalence of infectious organisms over a 30-year period. Surg Today. 2011; 41:346.

7. Johansen K, Devin J. Mycotic aortic aneurysms: a reappraisal. Arch Surg. 1983; 118:583–588.
8. Gomes MN, Choyke PL. Infected aortic aneurysms: CT diagnosis. J Cardiovasc Surg (Torino). 1992; 33:684–689.
9. Lee HL, Liu KH, Yang YJ, Kan CD. Bacteroides fragilis aortic arch pseudoaneurysm: case report with review. J Cardiothorac Surg. 2008; 3:29.
10. Jarrett F, Darling RC, Mundth ED, Austen WG. Experience with infected aneurysms of the abdominal aorta. Arch Surg. 1975; 110:1281–1286.

11. Miragliotta G, Del Gaudio T, Tajani E, Mosca A. Bacteroides thetaiotaomicron in posthysterectomy infection. Anaerobe. 2006; 12:276–278.

12. Rathinavelu S, Zavros Y, Merchant JL. Acinetobacter lwoffii infection and gastritis. Microbes Infect. 2003; 5:651–657.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download