Abstract
Chylothorax or chylous ascites is rare manifestation of tuberculosis. We report a case of simultaneous chylothorax and chylous ascites due to tuberculosis. A 17-year-old girl was admitted with fever, abdominal distention and dyspnea. Chest and abdominal computed tomography revealed bilateral pleural effusion, multifocal nodular consolidation on both lung fields and copious ascites and multiple necrotic lymphadenopathy in the abdominal cavity. Mycobacterium tuberculosis was isolated from sputum and pleural fluid. The patient was treated with anti-tuberculosis medication. Pleural effusion and ascites improved with the medication.
Chylothorax and chylous ascites, which is characterized by the presence of chyle in the pleural and peritoneal cavities, is an uncommon condition [1, 2]. Chylothorax or chylous ascites result from trauma or a malignant condition such as lymphoma. Tuberculosis (TB) can cause cylothorax or chylous ascites [3, 4, 5], but the concurrent occurrence of both manifestations associated with tuberculosis is extremely rare. We report a case of chylothorax and chylous ascites due to TB.
A 17-year-old girl was admitted to the Korean University Ansan Hospital with a one-month history of fever, abdominal distention and dyspnea. The patient had been treated in another hospital for 10 days but the cause of ascites and pleural effusion had not been discovered. She was transferred to our hospital for further care. On admission, body temperature was 38.6℃, heart rate was 146/min and body weight was 56 kg. On chest auscultation, breathing sound was decreased on both lower lung fields. Abdominal examination revealed shifting dullness and the lower extremities were severely edematous. A chest radiograph taken on admission showed diffuse fine nodular opacities in both lungs and bilateral pleural effusion. Chest computed tomography (CT) showed pleural effusion of both sides, more serious on the left side, and pericardial effusion and multifocal nodular consolidation on both lung fields (Fig. 1A). Abdominal CT showed a large amount of ascites with diffuse peritoneal thickening and multiple necrotic lymphadenopathy in the abdominal cavity, and enhanced wall thickening in the distal ileum and ascending colon (Fig. 1B). Initial laboratory results were as follows: white blood cell count 6,670/mm3, hemoglobin 6.7 g/dL, serum total protein 4.6 g/dL and albumin 2.2 g/dL. Thoracentesis and paracentesis were performed to verify the nature of fluid apparent in the chest and abdominal CT images.
Pleural fluid (Fig. 2A) was turbid and whitish-colored, with pH 8.0, protein level 3.1 g/dL, lactate dehydrogenase of 5,855 IU/L, triglyceride 133 mg/dL and adenosine deaminase 121.6 IU/L. The lactate dehydrogenase ratio of pleural fluid versus serum was 15, consistent with exudate. Ascites (Fig. 2B) was milk-like and turbid in appearance, pH 8.0, albumin level 0.5 g/dL, triglyceride 864 mg/dL and adenosine deaminase 14.8 IU/L. We diagnosed chylothorax and chylous ascites because the triglyceride level of pleural fluid and ascites were higher than 110 mg/dL and 200 mg/dL, respectively. Direct smear of pleural fluid and ascites for acid fast bacilli (AFB) was positive only in pleural fluid. So, finally we diagnosed the patient as having TB associated with chylothrax and chylous ascites on the basis of positive AFB stain and abdomen CT. Initially, the patient received anti-TB treatment with quadritherapy including isoniazid 300 mg/day, rifampin 600 mg/day, pirazinamide 1,500 mg/day and etambutol 1,200 mg/day. The patient also was put on a high protein and low fat diet with medium chain triglyceride. Mycobacterium tuberculosis was cultured from sputum and pleural fluid on 28th day from admission and the isolate was susceptible to all anti-TB medications. The patient improved continuously after anti-tuberculosis medication, and pleural effusion and ascites were slightly decreased on follow-up chest and abdomen X-ray. The patient was discharged on day 60 of hospitalization with ongoing anti-tuberculosis treatment.
Chlyothorax and chylous ascites is characterized by chyle in the pleural and peritoneal cavities produced by obstruction and disruption of the lymphatic channel [2]. The reported incidence of the combined occurrence of chylothorax and chylous ascites has varied from 9% to 55% of chylous effusion [5, 6]. The etiologies of chylothorax and chylous ascites can be nontraumatic and traumatic. The most common cause of nontraumatic chylous effusion is a malignancy, such as lymphoma or metastatic carcinoma [3, 4]. Other causes of nontraumatic chylous effusion include idiopathic, congenital anomaly, protein-losing enteropathy and TB [7, 8]. One case of systemic lupus erythematosus [7] and one case of Henoch-Schönlein purpura [9] accompanied by chylothorax and chylous ascites at a same time have been reported.
Our case did not have any other cause of chylothorax or chylous ascites except TB. The patient denied any history of trauma, chest and abdominal CT scan did not show any evidence of malignancy and repeated cytologic examinations of pleural effusion and ascites did not reveal malignant cells. Constrictive pericarditis, possible cause of chylothorax and chylous ascites, was not noted on the result of echocardiography. M. tuberculosis was isolated from sputum and pleural fluid, but not from ascites. Despite the low adenosine deaminase level in ascites and a negative reaction for TB upon polymerase chain reaction examination, an abdominal CT scan showed multiple necrotic lymphadenopathy in the abdominal cavity, which was suggestive of TB infection. As a result, we diagnosed chylothoax and chylous ascites due to TB infection. The mechanism of TB for development of chylothorax and chylous ascites may be related to the enlarged lymph node obstructing lymphatic duct or direct invasion to lymphtic system inducing inflammation [10, 11, 12, 13].
The mainstay of the treatment of chylothorax or chylous ascites is conservative measures and correcting the underlying causes. The patient received anti-TB medication and nutrition replacement with high protein and low fat meal with medium chain triglycerides.
During the first two weeks after this therapy, the patient still complained of dyspnea and abdominal discomfort, and repetitive paracentesis and thoracentesis was done to decompress the pleural and peritoneal space. After one month, a follow-up chest X-ray revealed decreased pleural effusion and abdominal distension was also improved. Although some case reports have described the use of octreotide in the management of chylothorax or chylous ascites [14, 15, 16, 17, 18, 19], our patient improved with a regimen of anti-TB medication, diet control and supportive care; octreotide was not used.
The concurrent occurrence of both manifestations associated with tuberculosis is extremely rare. With considering high prevalence of TB in Korea, however, TB should be considered in the differential diagnosis of chylothorax and chylous ascites.
Figures and Tables
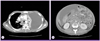 | Figure 1
Contrast-enhanced computed tomography (CT) scan images. (A) Chest CT scan showed left side dominant pleural effusion and multifocal mediastinal lymph node enlargement. (B) Abdominal CT scan showed large amount of ascites with diffuse peritoneal thickening and multiple necrotic lymphadenopathy in the abdominal cavity suggesting tuberculous peritonitis with lymphadenopathy. |
References
2. Aalami OO, Allen DB, Organ CH Jr. Chylous ascites: a collective review. Surgery. 2000; 128:761–778.

4. Press OW, Press NO, Kaufman SD. Evaluation and management of chylous ascites. Ann Intern Med. 1982; 96:358–364.

5. Romero S, Martín C, Hernandez L, Verdu J, Trigo C, Perez-Mateo M, Alemany L. Chylothorax in cirrhosis of the liver: analysis of its frequency and clinical characteristics. Chest. 1998; 114:154–159.
6. Nix JT, Albert M, Dugas JE, Wendt DL. Chylothorax and chylous ascites; a study of 302 selected cases. Am J Gastroenterol. 1957; 28:40–53. discussion, 53-5.
7. Lee CK, Han JM, Lee KN, Lee EY, Shin JH, Cho YS, Koh Y, Yoo B, Moon HB. Concurrent occurrence of chylothorax, chylous ascites, and protein-losing enteropathy in systemic lupus erythematosus. J Rheumatol. 2002; 29:1330–1333.
8. Cakir E, Gocmen B, Uyan ZS, Oktem S, Kiyan G, Karakoc F, Ersu R, Karadag B, Dagli T, Dagli E. An unusual case of chylothorax complicating childhood tuberculosis. Pediatr Pulmonol. 2008; 43:611–614.

9. Lee TH, Lee EY, Cho YS, Yoo B, Moon HB, Lee CK. Concurrent occurrence of chylothorax and chylous ascites in a patient with Henoch-Sch?nlein purpura. Scand J Rheumatol. 2003; 32:378–379.

10. Doerr CH, Allen MS, Nichols FC 3rd, Ryu JH. Etiology of chylothorax in 203 patients. Mayo Clin Proc. 2005; 80:867–870.

11. Antón PA, Rubio J, Casán P, Franquet T. Chylothorax due to Mycobacterium tuberculosis. Thorax. 1995; 50:1019.
12. Vennera MC, Moreno R, Cot J, Marin A, Sanchez-Lloret J, Picado C, Agusti-Vidal A. Chylothorax and tuberculosis. Thorax. 1983; 38:694–695.

13. Bond SJ, Guzzetta PC, Snyder ML, Randolph JG. Management of pediatric postoperative chylothorax. Ann Thorac Surg. 1993; 56:469–472. discussion 472-3.

14. Shah D, Sinn JK. Octreotide as therapeutic option for congenital idiopathic chylothorax: a case series. Acta Paediatr. 2012; 101:e151–e155.

15. Foo NH, Hwang YS, Lin CC, Tsai WH. Congenital chylothorax in a late preterm infant and successful treatment with octreotide. Pediatr Neonatol. 2011; 52:297–301.

16. Zhou DX, Zhou HB, Wang Q, Zou SS, Wang H, Hu HP. The effectiveness of the treatment of octreotide on chylous ascites after liver cirrhosis. Dig Dis Sci. 2009; 54:1783–1788.

17. Ijichi H, Soejima Y, Taketomi A, Yoshizumi T, Uchiyama H, Harada N, Yonemura Y, Maehara Y. Successful management of chylous ascites after living donor liver transplantation with somatostatin. Liver Int. 2008; 28:143–145.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download