Abstract
Actinomyces meyeri is rarely isolated in cases of actinomycosis. The identification of A. meyeri had historically been difficult and unreliable. With the recent development of 16S ribosomal RNA (16S rRNA) sequencing, Actinomyces species such as A. meyeri can be isolated much more reliably. A. meyeri often causes disseminated disease, which can be secondary to frequent pulmonary infections. A penicillin-based regimen is the mainstay of A. meyeri treatment, with a prolonged course usually required. Here, we report a case of pulmonary actinomycosis with brain abscess caused by A. meyeri that was initially thought to represent lung cancer with brain metastasis.
Actinomycosis is a chronic infection caused by organisms in the genus Actinomyces, with Actinomyces israelii being the most common etiologic agent [1]. The Actinomyces spp. are commensal organisms that make up the normal flora of the oropharynx, skin, gastrointestinal tract, and the female genital tract of humans [2]. Consequently, infection with actinomycetes commonly involves cervicofacial, thoracic, and abdominopelvic sites [2].
The identification of other Actinomyces spp. has historically been difficult and unreliable; thus, the potential for other species to cause similar clinical disease is largely unknown [2]. Recent developments in microbiological identification techniques, especially 16S ribosomal RNA (16S rRNA) sequencing, have identified other Actinomyces species such as A. meyeri, which are also being reported as human pathogens [1]. A. meyeri often causes pulmonary infection and shows a tendency for hematogenous dissemination [3]. Compared to the cases of disseminated actinomycosis due to A. meyeri that have been reported abroad [2, 3, 4, 5], only few cases of cerebral actinomycosis presenting as brain abscess have been reported in Korea [6, 7, 8]. Here, we report a case of pulmonary actinomycosis with brain abscess caused by A. meyeri resembling lung cancer with brain metastasis.
A 46-year-old man with no significant past medical history was admitted to the hospital with a 3-day history of headache and aphasia. He had smoked daily a pack of cigarettes for 20 years and consumed approximately five bottles of beer weekly. His temperature was 36.5℃, blood pressure was 138/90 mmHg, pulse rate was 79 beats/min, and respiration rate was 20 breaths/min. Although he had aphasia, he was alert. Examination of the mouth showed no evidence of poor dental hygiene or dental abscesses.
His leukocyte count was 11,800/mm3, with 72% neutrophils, 21% lymphocytes, and 6% monocytes. Blood tests revealed the following data: hemoglobin, 15.7 g/dL; platelets, 324,000/mm3; C-reactive protein, 0.18 mg/dL; and creatinine, 0.8 mg/dL. Serologic tests for Human Immunodeficiency Virus (HIV) and toxoplasmosis were negative. A chest radiograph showed a mass-like opacity in the left lower lobe (Fig. 1A), and computed tomography (CT) confirmed a 2 cm mass in the left lobe with a speculated border and peripheral subsegmental atelectasis (Fig. 1B). A magnetic resonance brain scan showed a 3.5 cm necrotic mass with peripheral rim enhancement in the left frontoparietal lobe, and a 0.6 cm, enhancing nodular lesion in the subcortical white matter of the left parietal lobe (Fig. 2). A presumptive diagnosis of lung malignancy with brain metastasis was made, and the patient was transferred to the department of pulmonology for a general work-up and staging of the lung cancer.
At the time of transfer, the patient's vital signs were stable, and his aphasia was improved by use of dexamethasone for the treatment of cerebral edema. A CT-guided percutaneous core-needle biopsy of the lung was performed; however, histological examination showed organizing pneumonia but no evidence of malignancy. Gram stainings and microbiological cultures of the biopsies for fungi, pathogenic bacteria, and mycobacteria remained negative. A second CT-guided percutaneous core-needle biopsy of the lung was performed after several days, and organizing pneumonia was pathologically confirmed again. A diagnosis of brain abscesses was considered, and a stereotatic biopsy was performed. The brain lesion appeared to be an abscess, and approximately 8 mL of yellowish and lightly greenish thick pus was obtained. The pus contained filamentous and non-filamentous Gram-positive rods on microscopic examination. The patient was treated with penicillin G (4 million units q 4 h) and metronidazole (500 mg q 8 h) intravenously for Actinomyces species and anaerobes. One week after starting treatment, the brain abscess cultures grew Actinomyces species, Propionibacterium acnes, and Fusobacterium nucleatum. The Actinomyces species was identified as A. meyeri using the 16S rRNA sequencing by Microgene Company (South Korea) and comparaing the sequencing data in the GenBank databases at the National Center for Biotechnology Information (NCBI). At first, the pus from the brain abscess was identified biochemically as bionumber 6760001000101, 85% A. meyeri by Vitek ANI card (bioMérieux, Marcy l'Etoile, France), which is a relatively low rate of certainty. Thus, 16S rRNA gene sequencing was performed for further identification. Using primers amplifying 9-806 bp (8FPL 5'-AGT TTG ATC CTG GCT CAG-3', 806R 5'-GGA CTA CCA GGG TAT CTA AT-3') and 515-1,390 bp (515FPL 5'-TGC CAG CAG CCG CGG TAA-3', 13B 5'-AGG CCC GGG AAC GTA TTC AC-3'), polymerase chain reaction (PCR) was performed according to previously published methods [9]. The purified PCR products were directly sequenced using the Big-Dye Terminator v3.1 Cycle Sequencing kit (Applied Biosystems, Life Technologies, Grand Island, NY, USA). According to a search of the Basic Local Alignment Search Tool (BLAST) database (http://www.ncbi.nlm.nih.gov/blast/) and eztaxon-e [10], the sequence of this isolate was a 99.9% (1,346/1,347 bp) match to the A. meyeri strain Prevot 2477B (GenBank accession no. NR_029286). As a secondary match, the sequence of the isolate was 98.1% (1,322/1,347) match to the A. odontolyticus strain F0309 (GenBank accession no. GQ131411). In line with the Clinical and Laboratory Standard Institute guidelines [11], the organism was identified as A. meyeri.
The patient received a 6-week course of penicillin G (4 million units q 4 h) and metronidazole (500 mg q 8 h) intravenously in the hospital, and was then discharged home to complete a 12-month course of oral amoxicillin (500 mg q 6 h).
Actinomycosis is a rare infection caused by anaerobic or microaerophilic Gram-positive bacilli belonging to the genus Actinomyces [12]. Most actinomycotic infections are polymicrobic and include other anaerobic or microaerophilic flora such as Actinobacillus actinomycetemcomitans, Fusobacterium spp., and Peptostreptococcus spp. [2]. These associated pathogens also belong to the human oropharyngeal flora. Hypothetical mechanisms by which associated bacteria may enhance the pathogenicity of Actinomyces species include reduced oxygen tension and anaerobiosis-induced inhibition of phagocytes [3]. Pulmonary infections are believed to occur due to aspiration of the organism from the oral cavity. In our case, A. meyeri infection involved the lung and brain. In addition, polymicrobial organisms, including Propionibacterium acnes and Fusobacterium nucleatum, were cultured from the brain abscess. Even though the patient had smoked a pack of cigarettes a day for 20 years, he did not have poor dental hygiene, nor did he drink alcohol heavily.
A. meyeri is a rare cause of actinomycosis in humans. It was first isolated from a patient with empyema by Meyer in 1911, who termed it Streptothrix. The microorganism was reclassified as Actinobacterium meyeri in 1938. The nomenclature was changed to Actinobacterium meyeri by Prevot in 1956 and subsequently to A. meyeri by Holderman in 1977 [13]. Although actinomycosis by A. meyeri was discovered more than 100 years ago, it has been accepted that members of the genus Actinomyces are difficult to accurately identify down to the species [14]. Historically, classification of Actinomyces species was based upon differentiation in a few phenotypic tests [15]. Biochemical reactions, colonial morphology, and gas-liquid chromatography were used to identify Actinomyces species. Recent advances in microbiological identification techniques, particularly 16S rRNA sequencing, have been used successfully to identify new Actinomyces species such as A. meyeri, A. europaeus, A. neuii, A. radingae, A. graevenitzii, A. turicensis, A. georgiae, A. funkei, A. lingnae, A. houstonesis and A. cardiffensis, which are also reported as human pathogens [1].
Actinomycosis of the central nervous system is rare. The source may be hematogenous, or it may develop through extension of oral-cervicofacial disease. In our case, the patient did not have poor dental hygiene, nor did he have oral-cervicofacial disease. Therefore, brain abscess caused by A. meyeri might have been from hematogenous spread.
Unlike A. israelii, which predominantly causes cervicofacial disease, the most common organ system involved by A. meyeri is pulmonary [1]. A. meyeri often causes disseminated disease, which can arise secondarily to frequent pulmonary infections [1]. According to one study, of 26 cases of A. meyeri. infection, 10 (38%) were disseminated (defined as the involvement of two or more distant organs) [3]. The propensity of A. meyeri to disseminate is difficult to explain because this organism does not differ from other actinomycetes in its microbiological characteristics [3]. It has been postulated that pulmonary infection is often the source of hematogenous dissemination [16], and this assumption is supported by the observation that in eight out of 10 cases of disseminated actinomycosis due to A. meyeri, pulmonary infection was present [3]. In the case reported here, it is presumed that hematogenous spread from the pulmonary lesions which led to disseminated actinomycosis with brain abscesses that were initially misdiagnosed as a metastatic tumor. Also, the finding that lung lesion had showed good response to penicillin treatment suggests that lung lesion be caused by A. meyeri.
Compared to the cases of disseminated actinomycosis due to A. meyeri that have been reported abroad [2, 3, 4, 5], only few cases of cerebral actinomycosis presenting as brain abscess have been reported in Korea [6, 7, 8]. With the exception of the first Korean report of a brain abscess caused by A. meyeri [6], all Korean cerebral actinomycosis cases were confirmed by the presence of sulfur granules on biopsy, which is a typical finding associated with actinomycosis [7, 8]. To our knowledge, this is the first report of disseminated A. meyeri infection.
Treatment of most patients with actinomycosis infection involves a prolonged course of antibiotics, accompanied by surgical or percutaneous drainage when indicated. Similar to other Actinomyces species, A. meyeri remains susceptible to several antibiotic classes. Penicillin continues to be the drug of choice for Actinomyces spp., including A. meyeri. Other antibiotics that have good in vitro activity and that have been used extensively with favorable clinical outcomes include erythromycin, tetracycline, doxycycline, and clindamycin. For localized disease, especially in the cervicofacial region, a relatively short course of oral penicillin (500 mg - 1 g q 6 h) for two months seems adequate [12]. For more extensive and disseminated disease, current expert opinion suggests intravenous penicillin (18-24 million units per day) for a period of two to six weeks, followed by oral penicillin or amoxicillin (500 mg q 6 h) [17]. The recommended duration of antibiotics therapy is six to twelve months, but this must be individualized based on the patient's clinical and radiologic response. The prognosis was favorable, even in patients with disseminated disease, including the brain [1]. In our case, the patient had undergone stereotatic aspiration of brain abscess, and he had received a 6-week course of penicillin G (4 million units q 4 h) and metronidazole (500 mg q 8 h) intravenously for A. meyeri and isolated anaerobes. After being discharged from the hospital, he was scheduled to complete a 12-month course of oral amoxicillin (500 mg q 4 h). At his five-month follow-up, he reported that his neurological symptoms resolved completely, and follow-up radiographs showed a marked decrease in the size of the lung nodules and brain lesion.
A case initially presumed as lung cancer with brain metastasis in a patient who presented with headache and aphasia was confirmed as an uncommon disseminated infection due to A. meyeri, which was accurately identified by 16S rRNA sequencing. Through surgical drainage of brain abscess and a 12-month, penicillin-based antibiotic regimen, the patient recovered completely. Disseminated infections with A. meyeri have rarely been reported in Korea. Here, we report a case of pulmonary actinomycosis with brain abscess caused by A. meyeri that was confirmed by 16S rRNA sequencing.
Figures and Tables
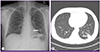 | Figure 1Initial chest roentgenogram shows mass-like opacity (arrow) in the left lower lobe (A). Initial chest CT shows a 2.2 cm mass (arrow) in the left lower lobe with a spiculated border and peripheral subsegmental atelectasis (B). |
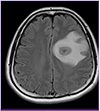 | Figure 2Initial brain MRI shows a 3.5 cm necrotic mass with peripheral enhancement in the left frontoparietal lobe, with perilesional edma and midline shifting. |
References
1. Fazili T, Blair D, Riddell S, Kiska D, Nagra S. Actinomyces meyeri infection: case report and review of the literature. J Infect. 2012; 65:357–361.

2. Colmegna I, Rodriguez-Barradas M, Rauch R, Clarridge J, Young EJ. Disseminated Actinomyces meyeri infection resembling lung cancer with brain metastases. Am J Med Sci. 2003; 326:152–155.

3. Apothéloz C, Regamey C. Disseminated infection due to Actinomyces meyeri: case report and review. Clin Infect Dis. 1996; 22:621–625.
4. Marty HU, Wüst J. Disseminated actinomycosis caused by Actinomyces meyeri. Infection. 1989; 17:154–155.

5. Kuijper EJ, Wiggerts HO, Jonker GJ, Schaal KP, de Gans J. Disseminated actinomycosis due to Actinomyces meyeri and Actinobacillus actinomycetemcomitans. Scand J Infect Dis. 1992; 24:667–672.

6. Song EH, Kwon HH, Jang EY, Kim MN, Lee SO, Kim YS, Woo JH. A case of brain abscess caused by Actinomyces meyeri and Haemophilus aphrophilus. Korean J Med. 2008; 74:Suppl 1. S144–S148.
7. Park YM, Won IS, Kim JI, Cho HJ, Seo JG, Kim JY, Kim EY, Park SH, Park YS, Seo YH, Cho YK. A case of central nervous system actinomycosis presenting as brain abscess. Infect Chemother. 2009; 41:249–252.

8. Ham HY, Jung S, Jung TY, Heo SH. Cerebral actinomycosis: unusual clinical and radiological findings of an abscess. J Korean Neurosurg Soc. 2011; 50:147–150.

9. Kolbert CP, Persing DH. Ribosomal DNA sequencing as a tool for identification of bacterial pathogens. Curr Opin Microbiol. 1999; 2:299–305.

10. Kim OS, Cho YJ, Lee K, Yoon SH, Kim M, Na H, Park SC, Jeon YS, Lee JH, Yi H, Won S, Chun J. Introducing EzTaxon-e: a prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int J Syst Evol Microbiol. 2012; 62:716–721.

11. Clinical and laboratory standards institute (CLSI). Interpretive criteria for identification of bacteria and fungi by DNA target sequencing; approved guideline. MM18-A. Wayne, PA: CLSI;2008.
13. Pordy RC. Lumpy jaw due to Actinomyces meyerii: report of the first case and review of the literature. Mt Sinai J Med. 1988; 55:190–193.
14. Hall V, Talbot PR, Stubbs SL, Duerden BI. Identification of clinical isolates of actinomyces species by amplified 16S ribosomal DNA restriction analysis. J Clin Microbiol. 2001; 39:3555–3562.

15. Holdeman LV, Cato EP, Moore WE. Anaerobe laboratory manual. 4th ed. Blacksburg, Virginia: Virginia Polytechnic Institute and State University;1977. p. 61–62.
16. Hennrikus EF, Pederson L. Disseminated actinomycosis. West J Med. 1987; 147:201–204.
17. Russo T. Agents of actinomycosis. Mandell, Douglas and Bennett's principles and practice of infectious diseases. 7th ed. Philadelphia, PA: Churchill Livingstone Elsevier;2010. p. 209–219.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download