Abstract
Spontaneous pneumothorax occurs in up to 35% of patients with Pneumocystis jirovecii pneumonia. However, spontaneous pneumomediastinum and pneumopericardium are uncommon complications in patients infected with human immunodeficiency virus, with no reported incidence rates, even among patients with acquired immunodeficiency syndrome (AIDS) and P. jirovecii pneumonia. We report a case of spontaneous pneumomediastinum, pneumopericardium, and pneumothorax with respiratory failure during treatment of P. jirovecii pneumonia in a patient with AIDS; the P. jirovecii infection was confirmed by performing methenamine silver staining of bronchoalveolar lavage specimens. This case suggests that spontaneous pneumomediastinum and pneumopericardium should be considered in patients with AIDS and P. jirovecii pneumonia.
Spontaneous pneumothorax has been recognized as a frequent complication in patients with Pneumocystis jirovecii pneumonia since it was first described in 1984 [1]. Spontaneous pneumothorax occurs in up to 35% of patients with P. jirovecii pneumonia. Spontaneous pneumothorax that occurs in patients with underlying lung diseases, such as chronic obstructive pulmonary diseases (COPD), cystic fibrosis, or P. jirovecii pneumonia, is termed secondary spontaneous pneumothorax and considered a potentially life-threatening condition. The incidence rate of spontaneous pneumothorax in patients infected with human immunodeficiency virus (HIV) was much higher than that in patients with COPD, especially in the pre-highly active antiretroviral therapy (HAART) era [2, 3]. However, spontaneous pneumomediastinum and pneumopericardium are uncommon complications in patients with HIV, with no reported incidence rates. Pneumopericardium in adults is most frequently iatrogenic, arising as a complication of invasive procedures such as mechanical ventilation or placement of central vein catheters [4]. The most frequent cause of pneumomediastinum is increased intrathoracic pressure, which may be caused by asthma or barotraumas, esophageal rupture, cavitating pneumonia (e.g., caused by Aspergillus fumigatus or Mycobacterium tuberculosis), or invasive procedures [5]. We report a case of spontaneous pneumomediastinum, pneumopericardium, and pneumothoraxin a patient infected with HIV who was receiving treatment for a P. jirovecii infection.
A 49-year-old man was admitted for fever and dyspnea associated with productive cough, sputum, and peripheral cyanosis. He was receiving 12.5 mg of thiazide and 40 mg of nifedipine once daily for hypertension. There was no past medical history of underlying pulmonary or cardiac disorders, and he was a non-smoker and non-alcoholic. He had lost approximately 6 kg in weight over the previous 3 months. On admission, his temperature was 38.3℃, with a respiratory rate of 20 breaths per minute, a pulse of 84 beats per minute, and a blood pressure of 110/70 mmHg. Auscultation of his lung fields revealed coarse breathing sounds with crackles in the right lower lung lobe, and electrocardiography revealed right bundle branch block. The complete blood cell count and blood chemistry on admission revealed a white blood cell count of 13,770/mm3 (neutrophils, 85.7%; lymphocytes, 9.5%; monocytes, 4.6%; eosinophils, 0.1%; basophils, 0.1%), hemoglobin level of 12.0 g/dL, platelet count of 195,000/mm3, and lactate dehydrogenase concentration of 589 U/L. Arterial blood gas analysis measured while breathing room air showed arterial oxygen partial pressure (PaO2) of 54.2 mmHg, arterial carbon dioxide partial pressure (PaCO2) of 30 mmHg, and oxygen (O2) saturation of 90.7%. Chest radiography on admission revealed diffuse bilateral reticular infiltrates in both lung fields (Fig. 1A). High resolution computed tomography (CT) scans showed diffuse ground glass opacity, ill-defined nodules with a clustered appearance, focal radiolucent lesions due to mosaic perfusion in both lungs, and subpleural consolidations in both lower lobes (Fig. 1B). Bronchiolitis obliterans organizing pneumonia was suspected, and 100 mg of hydrocortisone was administered intravenously twice daily. At the same time, 1 g of ceftizoxime and 500 mg of amikacin were administered intravenously twice and once daily, respectively, as the clinicians were unable to rule out a concurrent bacterial infection.
A pulmonologist performed a bronchoscopy on the 2nd day of hospitalization. During the procedure, oxygen saturation changes were monitored via pulse oximetry; despite oxygen supply via a nasal prong, hypoxemia (SaO2 < 85%) was frequently observed. We therefore discontinued the bronchoscopy without performing a transbronchial lung biopsy, but after collecting a bronchoalveolar lavage (BAL) sample from the right middle lobe. Following the recommendation of the pulmonologist who performed the bronchoscopy, we performed a HIV blood test and methenamine silver staining for the BAL specimens. The blood sample was positive for anti-HIV antibodies. Sputum and BAL specimens were negative for acid-fast bacilli on staining. Patient serum was also negative for cytomegalovirus IgM antibodies.
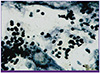
On the 8th day of hospitalization, a BAL sample was collected to test for P. jirovecii infection; microscopic examination showed foamy exudates positively stained with methenamine silver and also revealed cyst clusters morphologically consistent with P. jirovecii (Fig. 2). The patient was positive for HIV-1 on western blot analysis. In addition, quantitative real-time polymerase chain reaction showed an HIV viral load of 106,000 copies/mL, and the patient's CD4 T cell count was 17 cells/mm3.
High-dose intravenous trimethoprim/sulfamethoxazole was administered at a dose of 240/1,200 mg four times daily, and HAART was added to the treatment regimen on the 8th day of hospitalization. The patient also received 300/150 mg of zidovudine/lamivudine and 400/100 mg of lopinavir/ritonavir orally twice daily. On the 8th day of hospitalization, the patient experienced dyspnea that gradually intensified despite maintaining the oxygen saturation levels at 90-95%. His respiratory rate was approximately 30 breaths per minute and was accompanied by tachycardia. No additional medications or special studies were performed from the 9th to the 17th day of hospitalization because there were no apparent changes in vital signs or clinical symptoms.
On the 18th day of hospitalization, the patient's symptoms improved and his oxygen demand decreased to 4 L/min. Arterial blood gas analysis showed a PaO2 of 62.7 mm Hg, PaCO2 of 30.3mm Hg, and O2 saturation of 93.5%.
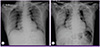
However, on the 19th day, chest radiography revealed free air in the mediastinum and band encircling the pericardium with right pneumothorax (Fig. 3A). We consulted a cardiologist and thoracic surgeon regarding the observed pneumothorax, pneumomediastinum, and pneumopericardium, who recommended that we maintain oxygen supplementation to facilitate pleural air resorption and perform regular follow-up chest radiography.
At approximately 6:00 AM on the 22nd day of hospitalization, the patient's oxygen saturation gradually decreased. Arterial blood gas analysis measured while breathing into a reserved mask bag showed a PaO2 of 52 mmHg, PaCO2 of 65 mmHg, and SaO2 of 82%. In response to respiratory acidosis, tracheal intubation was performed for mechanical ventilation. The pneumomediastinum and pneumopericardium did not significantly change in size, as observed via radiographic imaging (Fig. 3B). During mechanical ventilation, arterial blood gas analysis showed a PaO2 of 89 mmHg, PaCO2 of 29 mmHg, and O2 saturation of 95%.
Respiratory acidosis recurred at about 9:00 AM on the 25th day of hospitalization. SaO2 decreased and hypotension developed despite continued artificial ventilation. Additionally, the pulmonary lesion became aggravated. On the 28th day of hospitalization, the patient died from respiratory failure with persistent radiographic findings of pneumomediastinum and pneumopericardium.
Most patients with spontaneous pneumopericardium and pneumomediastinum recover spontaneously within 1 or 2 weeks unless a large amount of air is present [6, 7]. Pulmonary barotrauma, tuberculosis, severe cough, asthma, cocaine inhalation, chlorine gas exposure, emesis, and pneumonia can cause spontaneous pneumopericardium or pneumomediastinum [7, 8]. Herein, we describe a patient infected with HIV and P. jirovecii pneumonia who developed spontaneous pneumomediastinum, pneumopericardium, and pneumothorax with no history of trauma and no iatrogenic manipulations.
The bronchoscopy performed on the 2nd day of hospitalization could possibly have caused the observed pneumomediastinum and pneumopericardium. However, because we performed no invasive procedures such as tissue biopsy and collected only BAL fluid, the possibility of iatrogenic pneumomediastinum and pneumopericardium in this patient is low, considering the 0.1% incidence rate of pneumomediastinum and pneumopericardium after bronchoscopy. Flexible bronchoscopy is a common and safe outpatient procedure, with complications arising in less than 1% of cases. The case presented here details the occurrence of pneumomediastinum, pneumopericardium, pneumothorax, interstitial lung emphysema, and subcutaneous emphysema during bronchoscopy [9]. After a literature and chart review, these complications are not considered to be a direct result of the bronchoscopy [10, 11].
Pneumopericardium can be diagnosed via conventional chest radiography, CT, or echocardiography [12]. We did not perform chest CT as a means of further evaluation in this patient. However, every other paper reporting cases of pneumopericardium also used only chest radiography for diagnosis and treatment of pneumomediastinum and pneumopericardium; therefore,we do not believe that there is a limitation in our use of only chest radiography for diagnosis of pneumomediastinum and pneumopericardium in the present patient.
The pathophysiology of pneumomediastinum involves the pressure gradient between the alveoli and lung interstitium; increased pressure leads to alveolar rupture, resulting in air in the interstitial space flowing toward the mediastinum along the pressure gradient between the lung periphery and mediastinum [13].
Pneumomediastinum may be also due to leakage of air from cystic lesions (pneumatoceles) caused by proteases released by activated macrophages, as well as ischemic necrosis of vessels caused by P. jirovecii infection [14]. Gas-producing microorganisms present in the mediastinum as well as rupture of the mucosal barrier of the esophagus or tracheobronchial tree are considered possible mechanisms underlying the development of pneumomediastinum. Therefore, clinicians should be aware that pneumomediastinum could be observed in the clinical course of patients infected with HIV having P. jirovecii pneumonia [15]. However, it is not known how the presence of P. jirovecii contributes to the development of pneumomediastinum [16].
Pneumopericardium also develops because of a sudden increase in intra-alveolar pressure. Other possible causes such as trauma, presence of fistula from neighboring structures, or infection by gas-producing pathogens should be ruled out. An increase in intra-alveolar pressure due to bronchospasms or cough may provoke rupture of some alveoli, with ensuing pneumomediastinum resulting in air crossing the pericardial wall and leading to pneumopericardium [17]. The pathogenesis of pneumothorax secondary to P. jirovecii pneumonia in adults is unknown, but it is thought to be associated with rupture of subpleural cavities or emphysematous blebs [18].
Although Morris et al. reported decreased mortality rates in patients with AIDS who received HAART early in the acute phase of severe P. jirovecii pneumonia [19], immune reconstitution inflammatory syndrome (IRIS) following HAART might have contributed to the development of acute respiratory failure in this patient [20]. Furthermore, we cannot exclude the possibility that IRIS following HAART may have caused pneumomediastinum, pneumopericardium, and pneumothorax in this patient.
We present a patient with AIDS and P. jirovecii pneumonia who developed spontaneous pneumomediastinum, pneumopericardium, and pneumothorax and progressed to respiratory failure that resulted in death. Physicians should be aware that these conditions could becomelifethreatening, especially in patients with AIDS.
Figures and Tables
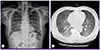 | Figure 1Radiological findings on admission of a 49-year-old man with Pneumocystis jirovecii pneumonia. (A) Chest radiography reveals diffuse infiltrate. (B) Computed tomography reveals diffuse ground glass opacities on both lung fields. |
References
1. Stringer JR, Beard CB, Miller RF, Wakefield AE. A new name (Pneumocystis jirovecii) for Pneumocystis from humans. Emerg Infect Dis. 2002; 8:891–896.
3. McClellan MD, Miller SB, Parsons PE, Cohn DL. Pneumothorax with Pneumocystis carinii pneumonia in AIDS. Incidence and clinical characteristics. Chest. 1991; 100:1224–1228.

4. Goodman PC. Radiographic findings in patients with acute respiratory distress syndrome. Clin Chest Med. 2000; 21:419–433. vii

5. Marco CA, Rothman RE. HIV infection and complications in emergency medicine. Emerg Med Clin North Am. 2008; 26:367–387. viii–ix.

6. Bartal N, Zaarura S, Marvan H. Spontaneous pneumopericardium and pneumomediastinum. Ann Otol Rhinol Laryngol. 1990; 99:1005–1006.

7. Agarwal MP, Giri S, Jain R, Sharma V. Spontaneous pneumopericardium in acute asthma. Int J Emerg Med. 2010; 3:141.

8. Horvat T, Savu C, Motas C, Tetu M. Pneumopericardium--complication of an unknown tuberculosis in a HIV positive patient. Eur J Cardiothorac Surg. 2004; 26:1043.

9. Barquero Romero J, Izquierdo Hidalgo J, Maciá Botejara E, Arrobas Vacas J, Pérez Miranda M. Spontaneous pneumopericardium in a patient with community-acquired pneumonia. Rev Esp Cardiol (Engl Ed). 2005; 58:227–229.

10. Choi WH, Hwang YM, Park MY, Lee SJ, Lee HY, Kim SW, Jun BY, Min JS, Shin WS, Lee JM, Koh YS, Jeon HK, Chung WS, Seung KB. Pneumopericardium as a complication of pericardiocentesis. Korean Circ J. 2011; 41:280–282.

11. Hasdiraz L, Oguzkaya F, Bilgin M, Bicer C. Complications of bronchoscopy for foreign body removal: experience in 1,035 cases. Ann Saudi Med. 2006; 26:283–287.

12. Iyer VN, Joshi AY, Ryu JH. Spontaneous pneumomediastinum: analysis of 62 consecutive adult patients. Mayo Clin Proc. 2009; 84:417–421.

13. Cho JY, Kim DM, Kwon YE, Yoon SH, Lee SI. Newly formed cystic lesions for the development of pneumomediastinum in Pneumocystis jirovecii pneumonia. BMC Infect Dis. 2009; 9:171.
14. Chow C, Templeton PA, White CS. Lung cysts associated with Pneumocystis carinii pneumonia: radiographic characteristics, natural history, and complications. AJR Am J Roentgenol. 1993; 161:527–531.

15. Kaji Y, Ohara G, Kagohashi K, Satoh H. Pneumomediastinum in a patient with Pneumocystis jirovecii pneumonia. Intern Med. 2012; 51:2251.

16. Moss S, Carey PB, Hind CR. Pneumocystis carinii pneumonia presenting with pneumomediastinum in an HIV-positive patient. Postgrad Med J. 1995; 71:96–97.

17. Abraham GE 3rd, Sumrall BH, Bowling MR. The air apparent: a rare complication during flexible bronchoscopy. Am J Med Sci. 2011; 341:243–245.

18. Schroeder SA, Beneck D, Dozor AJ. Spontaneous pneumothorax in children with AIDS. Chest. 1995; 108:1173–1176.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download