Abstract
Background
Carefully switching from intravenous to oral antibiotic therapy has shown to reduce treatment costs and lengths of hospital stay as well as increase safety and comfort in patients with infections. The aim of this study was to compare the clinical efficacy and safety between the patients treated with glycopeptides (case group), and the patients given oral antibiotics, as the initial or step-down therapy (control group), in the treatment of patients with methicillin-resistant Staphylococcus aureus (MRSA) infection.
Materials and Methods
A multicenter observational study was retrospectively performed in 7 teaching hospitals in Korea from January to December 2012. The study included adult patients (≥ 18 years) with infection caused by MRSA isolates, susceptible to clindamycin, erythromycin, and ciprofloxacin. The primary end point was treatment outcome, including all-cause mortality and switching of antibiotics. Drug-related adverse events and the lengths of hospital stay were also compared between the two treatment groups.
Results
During the study period, 107 patients (43 cases and 64 controls) with MRSA infections were enrolled from the participating hospitals. The most common sites of MRSA infection were skin and soft tissue (n = 28) and bone and joint (n = 26). The median Charlson comorbidity index (P = 0. 560), the frequency of severe sepsis (P = 0.682) or thrombocytopenia (P = 1.000), and median level of serum C-reactive protein (P = 0.157) at the onset of MRSA infections were not significantly different between the case and control groups. The oral antibiotics most frequently prescribed in the case group, were fluoroquinolones (n = 29) and clindamycin (n = 8). The median duration of antibiotic treatment (P = 0.090) and the occurrence of drug-related adverse events (P = 0.460) did not reach statistically significant difference between the two groups, whereas the total length of hospital stay after the onset of MRSA infection was significantly shorter in the case group than the control group [median (interquartile range), 23 days (8-41) vs. 32 days (15-54), P = 0.017]. In multivariate analyses, the type of antibiotic used was not an independent risk factor for treatment failure. The statistically significant factors associated with treatment failure included underlying hepatic diseases, prior receipt of antibiotics, and foreign body retention.
Although methicillin-resistant Staphylococcus aureus (MRSA) has been recognized as a predominant cause of nosocomial infection, it has become an increasingly common cause of community-associated infections [1, 2]. This suggests the spread of hospital-acquired MRSA isolates from hospitals into the community and vice versa [2]. Glycopeptides have traditionally been the drug of choice for the treatment of MRSA infections mainly because MRSA is resistant to other antimicrobial agents. However, some MRSA strains typically remain susceptible to a variety of antibiotics, including ciprofloxacin, trimethoprim/sulfamethoxazole (TMP/SMX), erythromycin, clindamycin, and tetracycline. Susceptibility to these antibiotics might be an independent predictor for a community-associated infection, in contrast to a hospital-acquired infection [3, 4].
As the incidence of MRSA infection continues to increase, there is mounting interest in potential alternatives to vancomycin or teicoplanin because of the spread of vancomycin-resistant enterococci (VRE). Particularly, skin and soft tissue infections that are not serious enough to require hospitalization, might be amenable to treatment with oral antibiotics in out-patients infected with MRSA isolates susceptible to oral antibiotics [5, 6, 7]. Furthermore, bone and joint infections, for which S. aureus is the most frequent causative organism, require long-term antibiotic therapy. If oral antibiotic agents are available for the treatment of bone and joint infections caused by MRSA isolates, it might reduce the pressure of antibiotic selection, reduce the emergence of VRE, and curtail the cost associated with intravenous antibiotics and prolonged hospitalization [8, 9, 10, 11].
Most clinicians consider intravenous infusion as the preferred route for administering antibiotics for serious infections. However, in recent years, several authors have suggested a regimen of relatively short intravenous therapy, followed by oral treatment for the remainder of the course as an antimicrobial stewardship program [12, 13, 14]. Although it may increase the rate of treatment failure, readmission, and death, an appropriate switch to treatment with oral antibiotics may allow early discharge, reduce treatment cost, risk of infection due to intravenous catheter, and the workload for the nursing staff, and increase comfort and mobility of the patients.
The previous study demonstrated that a favorable clinical cure rate was achieved with oral linezolid therapy when compared with intravenous vancomycin therapy in propensity score-matched patients with complicated skin and soft tissue infections caused by MRSA isolates [15]. However, a few data are available on which antibiotics can be used after intravenous glycopeptides in the management of patients with MRSA infections.
The aim of this study was to compare the clinical efficacy and safety of intravenous glycopeptide use alone versus oral non-β-lactam antibiotic use after intravenous antibiotics, in the treatment of patients with MRSA infection.
A multicenter observational case-control study was conducted in 7 teaching hospitals in the Republic of Korea from January to December 2012. The subjects comprised adult patients (≥ 18 years of age) with various infections due to MRSA isolates with community-acquired MRSA (CA-MRSA) phenotype [16, 17]. MRSA strains are susceptible to non-β-lactam antibiotics, which are used as oral agents after intravenous antibiotics, in the treatment of the following MRSA infections, except pneumonia: skin and soft tissue infections, bone and joint infection, urinary tract infections, bloodstream infections, intra-abdominal infection, cardiovascular infection, central nervous system infection and surgical site infection.
A case was defined as an adult patient who received non-β-lactam antibiotics as alternative oral agents to which MRSA is susceptible, as the initial or step-down therapy after glycopeptides therapy. A control was defined as an adult patient who received intravenous glycopeptides as the primary treatment for MRSA infections.
Only the first episodes of MRSA infection were included for analysis. Patients with polymicrobial infections were excluded to specifically evaluate the impact of antibiotic therapy for MRSA infection. During the study period, physicians treated the patients according to routine medical practice, without standardized interventions for the management of MRSA infection. The institutional review boards, prior to initiation of the study, approved the study protocol.
MRSA infection was considered present if one or more clinical cultures had positive results for MRSA isolates and if the clinical signs and course were consistent with MRSA infection; presence of fever, increased level of serum white blood cells (WBC) or serum C-reactive protein (CRP), and improvement of symptoms and infection signs after antibiotic therapy against MRSA isolates. Epidemiologically, MRSA infection was categorized as community-acquired, healthcare-associated, or nosocomial infection. Community-onset MRSA infections within 48 hours after hospital admission was considered healthcare-associated if during the preceding 12 months, the patient had any of the following: admission to other hospitals or healthcare facilities for more than 2 days, permanent indwelling catheters, surgery, dialysis, specialized home care, or visits at day hospitals. Infections occurring in patients at or after 48 hours of hospital admission were considered nosocomial [18]. Empirical antimicrobial therapy was defined as antibiotic treatment started before the causative organism and susceptibilities were investigated in clinical cultures. Therapy was classified as either appropriate or inappropriate based on the in vitro susceptibility of the organism to the antimicrobial agents used with an optimal dosing interval, within 48 hours of the index clinical culture collection. Prior antibiotic exposure was defined as administration of more than 3 doses of antibiotics, within 3 months before the occurrence of MRSA infection. The CA-MRSA isolate phenotype was defined as being susceptible to clindamycin, erythromycin, and ciprofloxacin [16, 17].
The primary end point was treatment failure, which was defined as a composite occurrence of all-cause mortality or switching of antibiotics due to adverse events or poor clinical response to antibiotic therapy. The secondary end points were mortality attributable to MRSA, hospital stay after onset of MRSA infection and relapse within 2 weeks after the termination of antibiotic treatment.
A standardized case report form was used to collect the clinical data of each patient from the participating hospitals. The parameters collected for this analysis included demographics, comorbid medical conditions, including Charlson comorbidity index [19], factors predisposing to infections, primary site of MRSA infection, diagnosis of severe sepsis or septic shock [20], hospital mortality, and microbiological data.
Bacterial identification and antibiotic susceptibility were performed at each study site using a VITEK II (bioMérieux, Hazelwood, MO, USA) or MicroScan Pos Combo Panel Type 6 system (Baxter Diagnostics, West Sacramento, CA, USA) in addition to test for inducible clindamycin resistance.
Categorical and continuous variables were expressed as percentage of a specific group or as median (Interquartile Range; IQR), respectively. Categorical variables were compared using Pearson's chi-square test when the expected frequency of each cell ≥5 or Fisher's exact test when the expected frequency of each cell <5. The continuous variables were compared using Mann-Whitney U test, because they were not normally distributed.
Multiple logistic regression analyses using the forward variable selection method were performed. All tests were two-tailed, and a P-value <0.05 was considered statistically significant. All of the analyses were performed with IBM SPSS Statistics version 20.0 (IBM Corporation, Armonk, NY, USA).
During the study period, 107 patients with infections due to MRSA isolates with CA-MRSA phenotype, were enrolled from the participating hospitals. The study included case patients who were administered non-β-lactam antibiotics, that are available as alternative oral agents (n = 43, 40.2%) and control patients who were given intravenous glycopeptides (n = 64, 59.8%) for MRSA infections. Antibiotic treatment in the case group was administered as an initial therapy (n = 16) [fluoroquinolones (n = 8), clindamycin (n = 3), TMP/SMX (n = 3), combination of ciprofloxacin and clindamycin (n = 1), and combination of ciprofloxacin and rifampin (n = 1)] or as a step-down therapy after glycopeptides therapy (n = 27) [fluoroquinolones (n = 21), clindamycin (n = 5), and combination of ciprofloxacin and rifampin (n = 1)]. The median duration of these oral antibiotic therapies was 14 days (IQR 10-44 days). In 27 patients who received a sequential oral antibiotic therapy after glycopeptide administration, the median duration of total antibiotic therapy and intravenous antibiotic therapy was 28 days (IQR 13-52 days) and 20 days (IQR 3-35 days), respectively.
The demographic and basal characteristics of the 107 patients are listed in Table 1. The most common primary site of MRSA infection was skin and soft tissue (n = 28, 26.2%), followed by bone and joint (n = 26, 24.3%), vascular catheter (n = 24, 22.4%), surgical wounds (n = 20, 18.7%), and others (n = 9, 8.4%). The univariate analyses revealed no significant differences in the primary site of infection between the case and control groups (Table 1).
The median Charlson comorbidity index was found to be 2 (IQR, 0-4) and univariate analyses showed no significant difference in Charlson comorbidity index between the case and control groups (Table 1). While underlying malignancies and renal diseases were significantly more common in the control group than in the case group (Table 1). Twenty-two patients (20.6%) had severe sepsis or septic shock, and there was no significant difference in the frequency of occurrence of severe sepsis, thrombocytopenia, and complicated conditions or median level of serum CRP at the onset of MRSA infections between the two treatment groups (Table 1).
Microbiological analysis was performed for all 107 MRSA isolates using an automated antimicrobial susceptibility testing system. In this study, over 90% of MRSA strains tested were susceptible to TMP/SMX (100%), fusidic acid (94.4%), and tetracycline (93.5%). Susceptibility to clindamycin (88.8%) and ciprofloxacin (87.9%) was also found to be better than to erythromycin (79.4%). All MRSA strains were susceptible to vancomycin and teicoplanin (Table 1).
The overall all-cause mortality and MRSA-related mortality were 15.9% (17/107) and 9.3% (10/107) respectively. There were no significant differences in the all-cause mortality and MRSA-related mortality between the two treatment groups. There was no significant difference observed in the duration of fever and the time period from onset of MRSA infection to normalization of WBC or CRP between the two treatment groups after initiation of the appropriate antibiotic therapy (Table 2). The median duration of antibiotic treatment in the case and control groups was 28 days (IQR, 13-52 days) and 18 days (IQR, 11-38 days) respectively (P = 0.090). There was no significant difference in the occurrence of drug-related adverse events between the two treatment groups (9.3% [4/43] vs. 14.1% [9/64], P = 0.460) (Table 2). However, length of hospital stay after onset of MRSA infection was significantly shorter in the case group than in the control group [23 days (IQR, 8-41 days) vs. 32 days (IQR, 15-54 days), P = 0.017) (Table 2). On the other hand, there was no significant difference in comparison of antibiotic treatment outcomes and related adverse events between the patients who received initial oral antibiotics and patients who received sequential oral antibiotics among the 43 patients in the case group (Table 3).
In the univariate analysis, predictors associated with treatment failure were found at the 5% significance level (Table 4). As the results, in the multiple logistic regression model, the antibiotic type (case group or control group) was not an independent risk factor for treatment failure in the patients with MRSA infections, regardless of variable selection (Table 5). The statistically significant factors associated with treatment failure included underlying hepatic diseases (odds ratio [OR]: 7.39; 95% confidence interval [CI], 1.48 to 36.84), prior receipt of antibiotics (OR: 4.01; 95% CI: 1.48 to 10.88), and foreign body retention (OR: 6.07; 95% CI: 1.64 to 22.37) (Table 4). The P-values for the Hosmer-Lemeshow goodness-of-fit test were greater than 0.05. Hence, each final model was a good fit for the data.
This multicenter study compared the clinical efficacy and safety of intravenous glycopeptide use alone versus oral non-β-lactam antibiotic use after intravenous antibiotics, in the treatment of patients with MRSA infection. This study showed that oral non-β-lactam antibiotic therapy, that MRSA isolates are susceptible to, can be considered as the initial or step-down therapy for the treatment of MRSA infection. Particularly, oral antimicrobial therapy significantly reduces the length of hospital stay.
This study covered diverse primary sources of MRSA infections, but excluded pneumonia due to its ambiguous aspect of diagnosis. The most common sites of MRSA infection were the skin and soft tissue (26.2%) and the bone and joints (24.3%). In the previous studies, CA-MRSA has primarily been described as a cause of skin and soft-tissue infections [21], acute ear infections [1], and necrotizing pneumonia [22, 23, 24]. Although these studies are not the large-scaled cohort studies for the patients with a single primary focus, these data demonstrated that CA-MRSA isolate can be considered as the causative microorganism in the patients with skin and soft tissue infections.
In an analysis of 107 MRSA strains, more than 90% of isolates retained susceptibility to TMP/SMX (100%), ciprofloxacin (87.9%), and clindamycin (88.8%). Two studies performed in Korea demonstrated that susceptibility rates to TMP/SMX, ciprofloxacin, and clindamycin were 96%, 46%, and 17% and 86.4%, 25%, and 38.2%, respectively [1, 2]. Compared to those of the previous studies, susceptibilities to ciprofloxacin and clindamycin were higher in our study. Based on those studies, the most favorable antibiotic for empirical therapy in patients suspected with CA-MRSA infection was TMP/SMX, similar to the results of our study. However, the Food and Drug Administration has not approved TMP/SMX for the treatment of MRSA infections. Although there are no randomized, controlled trials to support the use of this combination of antibiotic for patients, TMP/SMX has shown successful results in a small number of patients with CA-MRSA infections, and it exhibits bactericidal action against strains of CA-MRSA in vitro [25, 26, 27].
On the other hand, resistance rate of MRSA to ciprofloxacin and clindamycin differs among studies. Although this discrepancy may be owing to differences in methodology used for antimicrobial susceptibility testing, including test for inducible clindamycin resistance, the regional characteristics may also influence antimicrobial susceptibility patterns. Thus, the empirical choice of a specific antimicrobial agent should depend on local susceptibility patterns, which implies that the results of our study may not be representative of the larger population with CA-MRSA infections.
Despite an understanding of microbiological and epidemiological features, there is no clearly established definition of CA-MRSA. This has led to the use of several different definitions to describe CA-MRSA, including susceptibility to clindamycin or ciprofloxacin, non-multidrug resistance, presence of the staphylococcal cassette chromosome mec (SCCmec) IV or V, being Panton-Valentine leukocidin positive, causing community-onset infection combined with a lack of hospital-acquired factors and without previous history of being MRSA positive [17]. Of these, the definition based on antibiotic susceptibility may be useful for therapy in clinical practice if antibiotics, to which MRSA is susceptible, including non-β-lactam antibiotics, are made available for the treatment of patients with MRSA infection.
Multiple logistic regression analysis showed that the antibiotic type, based on the two treatment groups, was not a significant risk factor associated with treatment failure. These results suggest that oral antibiotics can be a safe and effective treatment option for MRSA infections. With an increase in the incidence of CA-MRSA infection, inexpensive oral agents commonly recommended for the treatment of CA-MRSA infections include clindamycin, doxycycline, minocycline, TMP/SMX, ciprofloxacin, rifampin, or fusidic acid as off-label antibiotic use [5, 6, 7, 8, 9, 10, 11, 28, 29]. However, clinical evidence supporting the efficacy of non-β-lactam antibiotics against MRSA is still insufficient. Furthermore, no studies have compared non-β-lactam antibiotics, which are available as alternative oral agents, with glycopeptides in adult patients with MRSA infection. Furthermore, the median duration of antibiotic treatment in the case and control groups was 28 days (IQR, 13-52 days) and 18 days (IQR, 11-38 days) respectively (P = 0.090).
Although the difference is not statistically significant, the median duration of antibiotic treatment in the case group was longer than that in the control group. This finding might result from the difference between out-patient setting in the case group and in-patient setting in the control group.
This study has some limitations. First, this study was not a randomized clinical trial as the physicians made the choice of antibiotic treatment. Therefore, the patients who were prescribed glycopeptides might have had a more severe infection. However, severity scales including Charlson comorbidity index, presence of severe sepsis or fever, and level of WBC or CRP were used to compare between the two groups. Second, the patients in the case group were often switched to different antibiotics if they showed treatment failure of primary antibiotic therapy against MRSA infection. Particularly, 27 case patients (62.8%) who were sequentially prescribed non-β-lactam antibiotics had already received intravenous glycopeptide therapy. Thus, these alterations may have influenced the treatment outcomes. In addition, this study failed to evaluate the clinical effect of the alternative antibiotics owing to the small number of study subjects. Lastly, the clinical efficacy of each antibiotic therapy may differ according to the primary site of MRSA infection. Although this study covered diverse site of MRSA infections, there is a need for future investigations to analyze patients with a single infection focus.
In conclusion, this multicenter study indicates that it is important to recognize that non-β-lactam antibiotics appear to be a useful alternative therapy against MRSA infections and may reduce the length of hospital stay, provided in vitro susceptibility against MRSA can be demonstrated. However, prospective large scale investigations are needed to further evaluate the efficacy and safety of these antibiotics.
Figures and Tables
Table 1
Comparison of demographic and clinical characteristics between the case and control groups involving 107 patients with MRSA infections
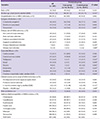
Table 2
Comparison of antibiotic treatment outcomes and related adverse events between case and control groups involving the 107 patients with MRSA infections

Table 3
Comparison of antibiotic treatment outcomes between the patients received initial oral antibiotics and patients received sequential oral antibiotics among the 43 patients in the case group

Table 4
Bivariate analysis of risk factors associated with treatment failure in the 107 patients with MRSA infections

IQR, interquartile range; MRSA, methicillin-resistant Staphylococcus aureus.
aP-values were obtained using Fisher's exact test.
bP-values were obtained using Mann-Whitney U test.
cCorticosteroid use was defined as the receipt of more than or equal to 20 mg/day prednisone equivalent for more than 5 days within the previous three months.
References
1. Kim ES, Song JS, Lee HJ, Choe PG, Park KH, Cho JH, Park WB, Kim SH, Bang JH, Kim DM, Park KU, Shin S, Lee MS, Choi HJ, Kim NJ, Kim EC, Oh MD, Kim HB, Choe KW. A survey of community-associated methicillin-resistant Staphylococcus aureus in Korea. J Antimicrob Chemother. 2007; 60:1108–1114.

2. Park SH, Park C, Yoo JH, Choi SM, Choi JH, Shin HH, Lee DG, Lee S, Kim J, Choi SE, Kwon YM, Shin WS. Emergence of community-associated methicillin-resistant Staphylococcus aureus strains as a cause of healthcare-associated bloodstream infections in Korea. Infect Control Hosp Epidemiol. 2009; 30:146–155.

3. Charlebois ED, Perdreau-Remington F, Kreiswirth B, Bangsberg DR, Ciccarone D, Diep BA, Ng VL, Chansky K, Edlin BR, Chambers HF. Origins of community strains of methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2004; 39:47–54.

4. Almer LS, Shortridge VD, Nilius AM, Beyer JM, Soni NB, Bui MH, Stone GG, Flamm RK. Antimicrobial susceptibility and molecular characterization of community-acquired methicillin-resistant Staphylococcus aureus. Diagn Microbiol Infect Dis. 2002; 43:225–232.

5. Khawcharoenporn T, Alan T. Oral antibiotic treatment for methicillin-resistant Staphylococcus aureus skin and soft tissue infections: review of the literature. Hawaii Med J. 2006; 65:290–293.
6. Enoch DA, Karas JA, Aliyu SH. Oral antimicrobial options for the treatment of skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus (MRSA) in the UK. Int J Antimicrob Agents. 2009; 33:497–502.

7. Barnes EV 2nd, Dooley DP, Hepburn MJ, Baum SE. Outcomes of community-acquired, methicillin-resistant Staphylococcus aureus, soft tissue infections treated with antibiotics other than vancomycin. Mil Med. 2006; 171:504–507.

8. Mathews CJ, Weston VC, Jones A, Field M, Coakley G. Bacterial septic arthritis in adults. Lancet. 2010; 375:846–855.

10. Sabol KE, Echevarria KL, Lewis JS 2nd. Community-associated methicillin-resistant Staphylococcus aureus: new bug, old drugs. Ann Pharmacother. 2006; 40:1125–1133.

11. Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004; 351:1645–1654.

12. Dryden M, Saeed K, Townsend R, Winnard C, Bourne S, Parker N, Coia J, Jones B, Lawson W, Wade P, Howard P, Marshall S. Antibiotic stewardship and early discharge from hospital: impact of a structured approach to antimicrobial management. J Antimicrob Chemother. 2012; 67:2289–2296.

13. Nathwani D, Eckmann C, Lawson W, Stephens JM, Macahilig C, Solem CT, Simoneau D, Chambers R, Li JZ, Haider S. Pan-European early switch/early discharge opportunities exist for hospitalised patients with methicillin-resistant Staphylococcus aureus complicated skin and soft-tissue infections. Clin Microbiol Infect. 2014; 03. 27. [Epub ahead of print].
14. Daver NG, Shelburne SA, Atmar RL, Giordano TP, Stager CE, Reitman CA, White AC Jr. Oral step-down therapy is comparable to intravenous therapy for Staphylococcus aureus osteomyelitis. J Infect. 2007; 54:539–544.

15. Itani KM, Biswas P, Reisman A, Bhattacharyya H, Baruch AM. Clinical efficacy of oral linezolid compared with intravenous vancomycin for the treatment of methicillin-resistant Staphylococcus aureus-complicated skin and soft tissue infections: a retrospective, propensity score-matched, case-control analysis. Clin Ther. 2012; 34:1667–1673.
16. O'Brien FG, Lim TT, Chong FN, Coombs GW, Enright MC, Robinson DA, Monk A, Saïd-Salim B, Kreiswirth BN, Grubb WB. Diversity among community isolates of methicillin-resistant Staphylococcus aureus in Australia. J Clin Microbiol. 2004; 42:3185–3190.
17. David MZ, Glikman D, Crawford SE, Peng J, King KJ, Hostetler MA, Boyle-Vavra S, Daum RS. What is community-associated methicillin-resistant Staphylococcus aureus? J Infect Dis. 2008; 197:1235–1243.

18. Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988; 16:128–140.

19. Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994; 47:1245–1251.

20. Longo DL, Fauci A, Kasper D, Hauser S, Jameson J, Loscalzo J. Harrison's principles of internal medicine. 18th ed. New York: McGraw-Hill;2012.
21. Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, McDougal LK, Carey RB, Talan DA. EMERGEncy ID Net Study Group. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006; 355:666–674.

22. Francis JS, Doherty MC, Lopatin U, Johnston CP, Sinha G, Ross T, Cai M, Hansel NN, Perl T, Ticehurst JR, Carroll K, Thomas DL, Nuermberger E, Bartlett JG. Severe community-onset pneumonia in healthy adults caused by methicillin-resistant Staphylococcus aureus carrying the Panton-Valentine leukocidin genes. Clin Infect Dis. 2005; 40:100–107.

23. Ko J, Chung DR, Park SY, Baek JY, Kim SH, Kang CI, Peck KR, Lee NY, Song JH. First imported case of skin infection caused by PVL-positive ST30 community-associated methicillin-resistant Staphylococcus aureus clone in a returning Korean traveler from the Philippines. J Korean Med Sci. 2013; 28:1100–1102.

24. Joo EJ, Chung DR, Ha YE, Park SY, Kang SJ, Kim SH, Kang CI, Peck KR, Lee NY, Ko KS, Song JH. Community-associated Panton-Valentine leukocidin-negative methicillin-resistant Staphylococcus aureus clone (ST72-MRSA-IV) causing healthcare-associated pneumonia and surgical site infection in Korea. J Hosp Infect. 2012; 81:149–155.

25. Kaka AS, Rueda AM, Shelburne SA 3rd, Hulten K, Hamill RJ, Musher DM. Bactericidal activity of orally available agents against methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 2006; 58:680–683.

26. Markowitz N, Quinn EL, Saravolatz LD. Trimethoprim-sulfamethoxazole compared with vancomycin for the treatment of Staphylococcus aureus infection. Ann Intern Med. 1992; 117:390–398.

27. Chen LF, Chastain C, Anderson DJ. Community-acquired methicillin-resistant Staphylococcus aureus skin and soft tissue infections: management and prevention. Curr Infect Dis Rep. 2011; 13:442–450.

28. DeLeo FR, Otto M, Kreiswirth BN, Chambers HF. Community-associated methicillin-resistant Staphylococcus aureus. Lancet. 2010; 375:1557–1568.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download