Abstract
Background
Rabies is an acute fatal viral disease generally transmitted from infected animals to humans through bites. It is distributed worldwide. The number of Korean people traveling to rabies-endemic countries and being bitten by infected animals has been increasing recently. Therefore, we investigated international travelers who received rabies post-exposure prophylaxis (PEP) at the National Medical Center (NMC) and compared the data with those of other clinics.
Materials and Methods
This study was a retrospective review of 106 patients who visited the International Travel Clinic of the NMC and received rabies PEP between July 2006 and December 2012. During that period, we used the Essen intramuscular regimen protocol. Complete rabies PEP was defined as 5 doses of rabies vaccination with or without rabies immunoglobulin (RIG) administration according to the World Health Organization guidelines.
Results
A total 106 cases documented within the period of 6 years were selected, including 10 children younger than 15 years and 96 older than 15 years. The mean age of the patients who received PEP was 33.4 years. Of the patients, 53 were male and another 53 were female. Most of the exposures occurred in Southeast Asia, predominantly from dog bites (71, 66.9%). The lower extremities were the most frequent site of exposure (37, 34.9%). All the patients began receiving rabies vaccination for prophylaxis after exposure, and 51 received rabies vaccination with RIG. Meanwhile, 74 cases (69.8%) initiated rabies vaccination overseas, but only 10 of them received RIG while overseas; the remaining 32 (30.2%) initiated rabies vaccination after returning to Korea. Within 7 days, all the children and 74 adults received their first rabies vaccination. Six adults initiated first rabies vaccination after 1 week. Eleven of the 106 patients stopped PEP before 5 doses, among whom 4 (1 child and 3 adults) discontinued vaccination after confirming that the biting animal remained healthy throughout 10 days of observation. None of the patients had been previously vaccinated against rabies.
Conclusions
Most of the overseas travelers who visited our clinic after being bitten by suspected rabid animals received appropriate rabies PEP. However, the interval between exposure and first rabies vaccination was often delayed. Tourists who plan to travel in rabies enzootic regions need to be aware that prompt initiation of PEP is important to reduce the risk for developing human rabies.
Along with the rapid increase in international travel, travelers are paying more attention to diseases that may be contracted during travel. Accordingly, the need for appropriate treatments and prevention has been emphasized. Rabies, a representative viral zoonosis, is a serious infectious disease that affects travelers visiting endemic regions. Once clinical symptoms develop and without special treatment methods, rabies becomes a fatal disease that leads to death within a few days [1].
According to a report from the World Health Organization (WHO), 55,000 people worldwide die annually because of rabies, mostly in Asia (31,000 people, 20,000 in India alone) and Africa (24,000 people). Among fatalities, 99.9% are due to rabid dog bites, with more than 80% reported to occur in rural areas. At present, more than 3 billion people in more than 100 countries live in regions with a risk of rabies virus infection [2]. In South Korea, human rabies cases were reported until 2004, while animal rabies cases have occurred continuously in Gyeonggi-do and northern Gangwon-do [3].
Although rabies is a fatal disease, complete protection is possible through appropriate management, specifically rabies vaccination and rabies immunoglobulin (RIG) vaccination, after bite and contact with rabid animals [1]. Therefore, it is important to reduce the risk of animal bites during travel to rabies-endemic areas; however, early adequate post-exposure prophylaxis (PEP) promptly after exposure is the most critical approach for decreasing death caused by rabies. More than 15 million people annually receive rabies PEP after animal-related injuries, and in the GeoSentinel Surveillance Network, animal-related injuries cause 1.4% of diseases among travelers [4]. In cases of domestically occurring animal bites, appropriate treatments and prevention can be administered relatively quickly because of convenient access to available medical services. However, for animal bites occurring during international travel, early PEP can be difficult depending on the local situation.
This study investigated the cases of people visiting the international travel clinics of the National Medical Center (NMC) for rabies PEP after the exposure to suspected rabid animals during international travel and examined the rabies PEP conditions of NMC by reviewing the previous international travel clinic studies in other countries.
A retrospective study was performed by reviewing the medical records of patients who visited the international travel clinics of the NMC for PEP after animal bites that occurred during international travel. Records from July 2006, when health insurance started covering rabies vaccine and human rabies immunoglobulin (HRIG) in Korea [5], to December 2012 were reviewed. On the basis of the disease code of the ICD-10 (KCD-6), patients with a diagnosis code of W54 (i.e., bitten or struck by dogs), W55 (i.e., bitten or struck by other mammals), or Z24.2 (i.e., need for immunization against rabies) were searched, and patients with records of animal bites during international travel were selected. Domestic animal bite patients and patients without information regarding the region of exposure occurrence were excluded from this study.
The following data of the 106 patients selected were analyzed: age, sex, vaccination history of the patient, animal species causing the bite, vaccination history of the animal, body site of the animal bite, region of exposure occurrence, interval between exposure and first rabies vaccination, local medical care and PEP, and management after visiting NMC.
On the basis of the Human Rabies Prevention and Control [5], published by the Korea Centers for Disease Control and Prevention (KCDC) in 2007, and the Essen regimen, adopted in the WHO PEP guideline [6], the evidence for appropriate PEP was classified as follows: patients who completed 5 doses of rabies vaccine, including local vaccination and vaccination after visiting our hospital, or those who completed 5 doses of rabies vaccine combined with HRIG were classified as having complete PEP; patients who stopped treatment without completing 5 doses of rabies vaccine were classified as having incomplete PEP. Of the patients with incomplete PEP, except for those who were confirmed to be healthy after the responsible animal was observed for 10 days, patients who decided to terminate treatment on their own without sound reasons were classified as having inappropriate PEP. Incubation period is a little different according to literature but one week is considered to be the minimum incubation period in the literature [7]. Therefore, the interval between exposure and the first rabies vaccination was categorized as starting rabies vaccination within 1 week and starting vaccination after 1 week.
Of the 371 patients who visited our clinic for rabies PEP between July 2006 and December 2012, 210 who had domestic animal bites and 55 who had no record of the region of exposure occurrence were excluded. The mean age of the resultant 106 patients was 33.4 years; 10 were children (i.e., <15 years old) and the other 96 were adults. The sex ratio in both the children and adults was 1:1. Regarding age distribution, more than half of the exposures occurred in patients in their 20s (37, 34.9%) and 30s (29, 27.4%; Fig. 1).
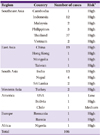
The regions of exposure occurrence were mostly in Southeast Asia; Thailand was the most common with 37 cases (34.9%), followed by China with 18 (16.9%), India with 13 (12.2%), and Indonesia with 12 (11.3%; Table 1).
Regarding the animal species causing the bite, dogs were the most common with 71 cases (66.9%), followed by monkeys with 26 cases (24.5%). There were 2 cases (1.8%) of bat-related exposure, which is an indication for the administration of rabies vaccination and RIG, because contact itself is included in the WHO category 3 (Fig. 2).
Regarding the body site of the animal bite, the lower extremities (37, 34.9%) were the most common, followed by the hands (19, 17.9%) and face (3, 2.8%). Four cases (3.7%) involved multiple injuries from more than two bites. Furthermore, there were 24 cases (22.6%) without information about the body site of the animal bite (Fig. 3). All facial bite injuries occurred in the children, and rabies vaccination was locally administered without RIG administration immediately after the exposure.
One or more doses of rabies vaccine were administered in all the cases; the first dose was administered locally and in our clinic in 74 (69.8%) and 32 cases (30.2%), respectively. Of the patients who started PEP in local medical centers, 13.5% (10/74) received rabies vaccination with RIG, whereas only 62.5% (20/32) received the first dose of rabies vaccine with RIG in our clinic.
The interval between exposure and the first rabies vaccination was known in 92 cases. The mean interval was 1.8 days. Among the children, all 10 patients started rabies vaccination within 7 days, while 6 (7.3%) of the 82 adults received their first vaccination more than 7 days after exposure (Table 2).
After post-exposure vaccination, complete PEP (i.e., 5-dose vaccination with or without RIG), based on the guidelines, was administered in 95 cases (89.6%). However, the 5-dose course was not completed in 11 cases (10.4%); vaccination was stopped in 4 cases after confirming a lack of abnormalities in the animal causing the bite observed for 10 days. Therefore, only 7 cases (6.6%) underwent incomplete appropriate prevention (Table 3). Of the 106 PEP cases, there were no records of preexposure vaccination prior to travel.
A review of previous studies from travel clinics in other countries suggests the present results are consistent with those reported in the literature. The median age was 30-35 years, and the percentage of children younger than 15 years was only 10%. Most studies report no significant sex differences. Bite injuries mostly occurred in Southeast Asia, especially Thailand. The main animal causing bite injuries was dogs, and the most common injury site was the lower extremities (Table 4) [9, 10, 11, 12, 13].
Regarding the age distribution of the patients in the present study, more than half were in their 20s or 30s. Meanwhile, there were relatively few children younger than 15 years, who are known to be most susceptible to rabies, with only 10 cases (9.4%). Among these cases, 5 cases, including the data of rabies vaccination-related studies, were published in the Korean Journal of Pediatric Infectious Diseases in 2013 [8]. This percentage is consistent with the results of a study in the UK but higher than that reported in Thailand and Nepal [9, 10, 11]. Only one study from New Zealand reports a higher child-to-adult ratio of 1:5, suggesting that children are at high risk group [12]. However, it is difficult to determine if certain age groups are high-risk groups because differences in age distribution are also influenced by differences in the absolute numbers of foreign travelers by age.
Exposure occurred most frequently in Southeast Asia, and bite injuries mostly occurred in rabies high-risk regions. Besides visiting China and Japan, Koreans visiting Southeast Asia is one of the reasons for the results, but other studies report that bite injuries occurred most commonly in Southeast Asia [9, 10, 11, 12, 13].
Dog bites were most common with 71 cases (67.0%), followed by monkey bites with 26 cases (24.5%). Previous studies also report remarkably high percentages of dog bites, in addition to monkey and cat bites [9, 10, 11, 12, 13]. In the present study, of the 26 cases of monkey exposure, 23 were concentrated in Thailand (13 cases) and Indonesia (10 cases), reflecting the regional distribution of monkey populations. A study in Bali, Indonesia, shows monkey-related injuries are more common than dog bites [14].
Although animal bites can occur when people disturb animals, there are many cases of unprovoked events, making the prevention of exposure difficult. However, the present results confirm the finding that animal bites commonly occur on the hands, which are used to feed and touch animals, and the lower extremities (i.e., the thighs, calves, and feet), which are at a similar height as the biting animals, mostly dogs; in addition, the face can also be a site of animal bites in children, who are relatively short. A study in Nepal that analyzed the association between mean age and injury site revealed that facial injuries were associated with a very low mean age compared to injuries at other sites [11]. The incubation period varies depending on the amount of inoculated virus or proximity of the bite to the central nervous system. In general, when a bite occurs near the head, the time to reach the central nerve system is shorter; this consequently shortens the incubation period because the virus directly invades the brain via the cranial nerves without passing the spinal cord [15, 16]. Therefore, if there is a chance of contacting animals in rabies-endemic areas, the hands and lower half of the body should be protected; meanwhile, the active care of children by adults is thought to be a method to reduce the occurrence of animal bites among children.
The KCDC has adopted the Essen regimen, which involves a total of 5 doses of rabies vaccine for PEP on days 0, 3, 7, 14, and 28, as well as 1 dose of RIG on day 0. In the case of delayed RIG administration, RIG can be administered within 1 week after the first vaccination regardless of when the animal bite occurred [17]. If more than 1 week after the first vaccination elapses, RIG is not administered because it negatively influences active immune response [18].
There were 74 patients (69.8%) who started rabies vaccination for PEP in local medical centers, but RIG was administered simultaneously in only 10 patients. The other 64 patients were vaccinated with RIG in our clinic within 1 week after the first vaccination. As shown in studies from clinics in the UK and Thailand, such low local RIG vaccination rates are expected because of the complex issues of relatively low availability of RIG compared with rabies vaccine, a lack of patient understanding about rabies PEP, and cost [9, 10].
According to the WHO, PEP is recommended with respect to 3 categories (i.e., categories I, II, and III) according the contact and exposure type to suspect rabid animals. RIG administration is recommended only for category III cases [19]. However, in this study, it was difficult to determine the category of most cases at the time of exposure from the medical records; thus, which patients had indications for RIG administration was practically unknown. When it is difficult to determine the category of a rabies case on the basis of the characteristics of the animal bite or accurately take the history of the exposure, active treatments are recommended, especially in children, because there is a possibility of implementing unfavorable PEP plans. Therefore, the notably high percentage of RIG vaccinations in our clinic compared with local clinics is considered to be a sound rabies PEP.
Verorab™(Sanofi Pasteur, Lyon, France) (rabies vaccine) and KamRAB™(Kamada, Rehovot, Israel) (HRIG) are utilized in Korea and regulated by the Korea Orphan Drug Center; their costs per vial are around US $60 and US $220, respectively. For adults, more than 4 or 5 vials should be utilized for HRIG on the basis of body weight, which costs more than US$1000. However, health insurance began covering these medicines in July 2006. Therefore, a copayment of only 30% is required [5]. Reduced economic burden is believed to be why active treatment is possible.
Even though the incubation period of rabies is generally approximately 1-3 months, it ranges from less than 1 week to more than 1 year [7, 15]. Another study reports it to range from 12 days to more than 2 years [14]. In the present study, the median time from exposure to rabies PEP was 1.8 days, which is not considerably different from other studies. Pediatric patients visited local hospitals or our clinic for medical care and rabies prophylaxis after being exposed to suspected rabid animals. However, some adults delayed their first visit to medical centers by more than 1 week to 1 month after exposure. A few adults were passively unaware of rabies prophylaxis after animal bites. Meanwhile, children, who do not have the right to make decisions about medical treatment for themselves, were still able to start appropriate early medical care and PEP because of the proactive attitude of their parents.
In the present study, most of the patients who started PEP completed the 5-dose vaccination regimen. Although only 7 patients (6.6%) received inappropriate PEP, there are likely more patients that had self-treatment after exposure or terminated treatment after local primary treatments without visiting our clinic.
Even though our international travel clinics occasionally perform preexposure vaccination prior to travel, no cases of preexposure vaccination were recorded; this contrasts with other studies, which confirm a few cases receiving preexposure vaccination. The main reason for the lack of preexposure vaccination not only in Korea but in most other counties is the high cost of the rabies vaccine. As complete protection is possible with appropriate treatment and PEP even after an animal bite, preexposure prophylaxis with the expensive rabies vaccine before traveling is controversial [20]. However, preexposure prophylaxis is recommended for patients at high risk of rabies infection, such as those working in rabies diagnostic or research laboratories, veterinarians, animal handlers (including bat handlers), animal rehabilitators, wildlife officers, children younger than 15 years of age living in or traveling to high-risk areas, and people who lack immediate and appropriate local medical care [21, 22].
In our clinic, the number of travelers opting for rabies preexposure prophylaxis is increasing annually. Recommending preexposure prophylaxis can decrease the risk of rabies occurrence possibly because of the low local RIG vaccination rate.
Rabies vaccination of animals causing the bite injuries, which is an important factor for confirming if appropriate PEP has been performed, was only confirmed in 14 cases. Most animals were stray dogs or animals without owners or records. Because the local situation at the time of exposure and the severity of bite injury were not recorded in most cases, category classification could not be performed. Therefore, it was difficult to determine if appropriate treatment according to guidelines had been administered. In addition, the lack of records of the location of bite injuries in 55 patients, which made it unknown whether the bite injury occurred domestically or overseas, is another limitation of this study.
In conclusion, this study confirms that exposure is most frequent on the lower extremities owing to dog bites in Southeast Asia among all the age groups, which is concordant with previous studies. In addition, the children had more bite injuries on the face and more active treatments administered immediately after the bite injury than the adults. Appropriate PEP was performed in most cases, but some cases lacked local RIG vaccination and delayed PEP until the first vaccination after bite injuries. In pre-travel clinical consultations, the healthcare advisors of the International Travel Clinic should highlight the need for prompt initiation of PEP after bite injuries for tourists who plan to travel to rabies-endemic regions. Moreover, active preexposure prophylaxis should be recommended when traveling to regions where it is difficult to obtain RIG.
Figures and Tables
 | Figure 3Number of exposed travelers according to the body site of the animal bite.
aExposure occurred at 2 or more body sites.
bOlder than 15 years.
cAged 15 years or younger.
|
Table 3
Analysis of post-exposure prophylaxis after animal bites

PEP, post-exposure prophylaxis; HRIG, human rabies immunoglobulin.
aFive doses of rabies vaccination with or without HRIG administration.
bFewer than 5 doses of vaccination with or without HRIG administration.
cRabies vaccination was stopped after confirming the biting animal remained healthy throughout a 10-day observation period.
References
1. Meslin FX. Rabies as a traveler's risk, especially in high-endemicity areas. J Travel Med. 2005; 12:Suppl 1. S30–S40.

3. Han MY, Cho JE, Seo SY, Jeong YE. Epidemiological features of animal bite cases occurred in highly risk areas of rabies in Korea, 2005 to 2008. In : 20th International Conference on Rabies in the Americas; 2009. p. 45–46.
4. Gautret P, Schwartz E, Shaw M, Soula G, Gazin P, Delmont J, Parola P, Soavi MJ, Matchett E, Brown G, Torresi J. GeoSentinel Surveillance Network. Animal-associated injuries and related diseases among returned travellers: a review of the GeoSentinel Surveillance Network. Vaccine. 2007; 25:2656–2663.

5. Korea Centers for Disease Control and Prevention. Human rabies prevention and control. 2007. Accessed 21 June 2013. Available at: http://www.cdc.go.kr/CDC/cms/cmsFileDownload.jsp?fid=51&cid=9803&fieldName=attach1&index=1.
6. World Health Organization. Recommendations on rabies post-exposure treatment and the correct technique of intradermal immunization against rabies. HYPERLINK "https://www.google.co.kr/url?q=http://www.who.int/&sa=U&ei=-koqU-OdLM2-kQXk3IDwAQ&ved=0CCAQFjAA&sig2=3oSkKgZhiN-ztohGRryzog&usg=AFQjCNHzge5cWim6pocYzhODW-P-6LA70Q" \t "_blank". Accessed 21 June 2013. Available at: http://www.who.int/rabies/en/WHO_recommendation_post_exp_treatment.pdf.
7. Madhusudana SN, Sukumaran SM. Antemortem diagnosis and prevention of human rabies. Ann Indian Acad Neurol. 2008; 11:3–12.

8. Noh JC, Park HM, Park JH, Won YK, Lee CH, Kim JY. Five year experience of preexposure and postexposure rabies prophylaxis in Korean children at the National Medical Center. Korean J Pediatr Infect Dis. 2013; 20:9–16.

9. Sibunruang S, Tepsumethanon S, Raksakhet N, Tantawichien T. Rabies immunization of travelers in a canine rabies endemic area. J Travel Med. 2013; 20:159–164.

10. Wijaya L, Ford L, Lalloo D. Rabies postexposure prophylaxis in a UK travel clinic: ten years' experience. J Travel Med. 2011; 18:257–261.

11. Pandey P, Shlim DR, Cave W, Springer MF. Risk of possible exposure to rabies among tourists and foreign residents in Nepal. J Travel Med. 2002; 9:127–131.

12. Shaw MT, O'Brien B, Leggat PA. Rabies postexposure management of travelers presenting to travel health clinics in Auckland and Hamilton, New Zealand. J Travel Med. 2009; 16:13–17.

13. Gautret P, Shaw M, Gazin P, Soula G, Delmont J, Parola P, Soavi MJ, Brouqui P, Matchett DE, Torresi J. Rabies postexposure prophylaxis in returned injured travelers from France, Australia, and New Zealand: a retrospective study. J Travel Med. 2008; 15:25–30.

14. Susilawathi NM1, Darwinata AE, Dwija IB, Budayanti NS, Wirasandhi GA, Subrata K, Susilarini NK, Sudewi RA, Wignall FS, Mahardika GN. Epidemiological and clinical features of human rabies cases in Bali 2008-2010. BMC Infect Dis. 2012; 12:81.

15. Anonymous. Rabies vaccines. WHO position paper. Wkly Epidemiol Rec. 2010; 85:309–320.
16. Dimaano EM, Scholand SJ, Alera MT, Belandres DB. Clinical and epidemiological features of human rabies cases in the Philippines: a review from 1987 to 2006. Int J Infect Dis. 2011; 15:e495–e499.

17. Korea Centers for Disease Control and Prevention. Epidemiology and prevention of vaccine-preventable disease. 2011. Accessed 21 June 2013. Available at: http://www.cdc.go.kr/CDC/cms/cmsFileDownload.jsp?fid=51&cid=9943&fieldName=attach1&index=1.
18. Khawplod P, Wilde H, Chomchey P, Benjavongkulchai M, Yenmuang W, Chaiyabutr N, Sitprija V. What is an acceptable delay in rabies immune globulin administration when vaccine alone had been given previously? Vaccine. 1996; 14:389–391.

19. World Health Organization. WHO guide for rabies pre and post-exposure prophylaxis in humans. Accessed 21 June 2013. Available at: http://www.who.int/rabies/PEP_prophylaxis_guidelines_June10.pdf.
21. Anonymous. WHO expert consultation on rabies. World Health Organ Tech Rep Ser. 2005; 931:1–88.
22. World Health Organization. International travel and health. Geneva, Switzerland: WHO Press;2011.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download