Introduction
1. Background and purpose
2. Scope
3. Guideline development methods
1) Establish a committee for developing the guidelines
2) Define the scope of the guidelines
3) Framing key questions
4) Searching for evidence
5) Writing the guidelines and clarifying the strength of the recommendations
Practice guidelines
1. Osteomyelitis
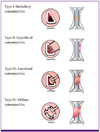
1) Epidemiology and classification
2) Distribution of causative pathogens
3) Clinical findings
4) Diagnosis
Acute hematogenous osteomyelitis occurs with rapid onset within a few days, and is usually accompanied by pain or tenderness over the affected bone and generalized symptoms such as fever or chills. The onset of chronic osteomyelitis is insidious. Symptoms of chronic osteomyelitis are subtle, but include mild generalized symptoms, pain or tenderness over the affected bone for long periods, and sinus tract involvement. Osteomyelitis must be suspected in such cases (AIII).
After an intensive review and physical examination, plain radiographs and blood tests including complete blood count (CBC) with differential counts, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) levels must be performed to confirm diagnosis (AIII).
Magnetic resonance imaging (MRI) is considered the best modality for the early detection of osteomyelitis (AI). Three-phase bone scintigraphy can be used for patients for whom computed tomography or MRI approaches are difficult (BIII).
In principle, cultures of blood, abscess, and bony tissue specimens must be obtained before the institution of antimicrobial agents (AIII).
Swab culture results from sinus discharge are often inaccurate for the detection of causative microorganisms, and thus cannot be relied upon. Culture from surgical or percutaneous bone biopsy is effective and should be performed (AII). Along with bony tissue culture, a histopathological examination can be performed to increase diagnostic sensitivity (BII).
5) Treatment
(1) General principles of treatment (Fig. 2)
In cases of acute osteomyelitis, appropriate antimicrobial agents should be given promptly to limit bacteremia, bone necrosis and bone destruction (AI).
Surgical treatment should be considered in cases of acute osteomyelitis when there is abscess formation or radiologic evidence of necrosis, or when the patient does not respond to antimicrobial agents (AII).
A multidisciplinary team approach is needed for the treatment of chronic osteomyelitis (AIII). Surgical interventions including adequate debridement of necrotic tissue, stabilization of bony structures, management of dead space, and reconstruction of soft tissue are needed. It is essential that the selected antimicrobial agents are appropriate for the isolated organism and that dosage and treatment duration is adequate.
Patient factors, such as improving nutritional state, stopping smoking, controlling glucose levels, and restoring blood flow, should be optimized as a part of treatment in patients with chronic osteomyelitis (AIII).
Surgical modalities and duration of antimicrobial agents are determined based on the Cierny-Mader's classification. In general, we recommend antimicrobial treatment of 4-6 weeks after the last major debridement. However, treatment must be tailored according to the stage and condition of the individual patient (AI).
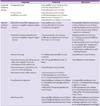
(2) Antimicrobial therapy (Table 6)
-
Empirical antimicrobial agents must be administered after obtaining cultures from blood, abscess, and bone tissue specimens. Prior to obtaining a definitive Gram stain and culture result, the clinician must select an appropriate antimicrobial agent considering the epidemiologic factors of the community, local susceptibility rates, the origin of infection (community or hospital), the general condition of the patient, and the primary cause of osteomyelitis.
Definitive antimicrobial therapy must be tailored according to the Gram stain results, the susceptibility of the isolated microorganism, and the degree of bone penetration (AII). -
Empirical antimicrobial treatment
1) In cases of community-acquired osteomyelitis, nafcillin or cefazolin is recommended as an empirical agent, given that the most commonly isolated organism is methicillin-susceptible S. aureus (MSSA) (AI).2) If gram-negative bacteria cannot be ruled out as the causative agents in patients with community-acquired osteomyelitis, ceftriaxone can be combined with nafcillin or cefazolin for coverage of both staphylococci and gram-negative pathogens (CIII).3) In cases of healthcare-associated or hospital-acquired osteomyelitis, and in cases that have responded poorly to antistaphylococcal treatment, vancomycin or teicoplanin can be considered to cover for MRSA (CIII). -
Antimicrobial treatment for specific organisms
1) Nafcillin or cefazolin should be administrated to treat osteomyelitis caused by MSSA (AI).2) Vancomycin is not recommended for the treatment of osteomyelitis caused by MSSA because of the high rate of recurrence of MSSA (AII).3) Either vancomycin or teicoplanin is recommended as first-line therapy for MRSA osteomyelitis (BII).4) The trough concentration of vancomycin should be 15-20 µg/mL when treating MRSA osteomyelitis (BIII). -
Role of combination antimicrobial treatment and switching to oral agents
1) Rifampin-containing regimen at early stage is generally not recommended for the treatment of osteomyelitis (CIII).2) Combinations regimens can be used as oral step-down treatments for osteomyelitis caused by S. aureus, if susceptible. They include rifampin + ciprofloxacin, rifampin + levofloxacin, and rifampin + trimethoprim/sulfamethoxazole (BII).3) Oral first-generation cephalosporin agents such as cefadroxil, cephalexin, and cefradine are generally not recommended (CIII).4) For the treatment of osteomyelitis caused by P. aeruginosa, monotherapy with a quinolone is not adequate because of the high risk for the development of resistance during the high bacterial burden that exists in the initial stages of disease. Therefore, combination therapy with a β-lactam agent and an aminoglycoside should be initiated at early stage of treatment (BIII).
(4) Treatment of vertebral osteomyelitis
Vertebral osteomyelitis is associated primarily with hematogenous monobacterial infection, and requires appropriate selective antimicrobial treatment (AII).
Indications for surgery include obtainment of specimen for microbiological and histological diagnosis, resolution of compression of neural elements, stabilization of instability due to extensive bone destruction, prevention or correction of biomechanical deformity such as severe kyphosis, drainage of clinically significant abscesses, or management of intractable pain (BIII). Spinal cord compression due to epidural abscess is a surgical emergency; the abscess must be surgically decompressed within 24-36 hours of the development of neurologic deficits (AI).
The recommended duration of antimicrobial treatment is usually 6-12 weeks. However, treatment must be individualized for each patient according to clinical response, course of improvement, antimicrobial susceptibility of the causative organism, and the presence or absence of an implant (BIII).
2. Septic arthritis
1) Epidemiology
2) Distribution of causative pathogens
3) Clinical opinions
4) Diagnosis
-
Joint fluid analysis
1) In patients with suspected septic arthritis, joint fluid analysis should be immediately conducted before antimicrobial agents are administered (AII).2) Joint fluid should be collected for Gram staining and culture, and the culture test should be performed using liquid agar, or after centrifuging, agar plates (AII).3) Septic arthritis patients taking warfarin should still undergo joint fluid analysis (BIII).4) Total white blood cell (WBC) and differential counts in joint fluid should be checked (AII) (Table 9).5) To identify the causative pathogens, a polymerase chain reaction (PCR) can be conducted (CIII).6) In cases of suspected tuberculosis, an acid-fast bacilli stain, culture, and PCR test can be conducted (BIII).7) In cases of suspected fungal infection, fungal infection tests can be conducted (BIII).8) To confirm the crystallization of joint fluid, polarizing microscopy should be performed. The joint fluid should be stored at room temperature (AII). If crystallization of joint fluid is confirmed, septic arthritis still cannot be ruled out. -
Laboratory tests
1) Uric acid level analysis is not helpful when diagnosing gout or septic arthritis (BII).2) Before antimicrobial agents are administered, a blood culture test should be conducted (AII).3) WBC count, CBC, ESR, and CRP tests should be conducted (AII). However, even if WBC count, ESR, and CRP are not increased, septic arthritis may still be diagnosed. -
Radiology examination
1) Septic arthritis cannot be diagnosed by plain radiography of infected joints; however, such methods can be used for distinguishing septic arthritis from other diseases (BII).2) MRI is helpful for checking for osteomyelitis and skin and soft tissue infection in areas near septic arthritis, and for determining the need for surgery (BII).3) A bone scan can be used as an additional method for confirming septic arthritis (BIII). -
Tissue biopsy and culture
A biopsy and tissue culture should be obtained during irrigation or curettage (AIII).
5) Treatment (Table 10, 11, 12)
-
If septic arthritis is suspected, joint fluid and blood culture samples should be collected and empirical - antimicrobial agents should be instituted, and then switched to selective antimicrobial agents according to the results of the Gram stain, as follows:
1) In cases without risk factors, cefazolin, ampicillin/sulbactam, or nafcillin should be used.2) In cases with risk factors for MRSA infection, vancomycin or teicoplanin should be used.3) In cases with risk factors for gram-negative bacterial infection, ceftriaxone should be used.4) In cases with risk factors for gonococcal infection, ceftriaxone should be used. Bactericidal drugs should be chosen for empirical therapy (BII).
As soon as septic arthritis is diagnosed, sufficient draining should be conducted immediately (AII).
Early joint aspiration is performed for septic joints. After 24 to 48 hours that joint aspiration is done, repeated procedure of joint aspiration and antimicrobial agents therapy will be ineffective. In this case, surgical procedures will be necessary. If joint aspiration is unavailable, surgical procedure will be necessary (AIII).
According to the antibiotic susceptibility of the cultivated strain, targeted antimicrobial agents should be maintained or changed (AII).
In general, the total antimicrobial agents treatment period should be about 4-6 weeks, with injectable antimicrobial agents being adminstered for at least 2 weeks. After 2 weeks, treatment may be switched to oral antimicrobial agents if the symptoms improve (CIII).
By monitoring the results of ESR and CRP tests as indicators of acute infection as well as the clinical manifestations of joint symptoms, the end-point for antibiotic therapy can be determined (CIII).
If the culture result is negative and septic arthritis remains suspected, antimicrobial agents treatment should be maintained. Empirical antimicrobial agents should be continuously administered for as long as joint responses improve. Antimicrobial agents treatment can be terminated based on joint symptoms, ESR, CRP level, and clinical manifestations (BII).




 PDF
PDF ePub
ePub Citation
Citation Print
Print














 XML Download
XML Download