Abstract
Psoriasis is a chronic inflammatory skin disease that involves immune-mediated cutaneous inflammation and keratinocyte hyperproliferation. Psoriasis in patients with HIV responds poorly to treatment and has a high morbidity rate, thus posing a challenge to clinicians. Until now, there have been no documented cases of acitretin therapy for HIV-associated psoriasis in Korea. Here, we report a case of safe and successful therapy with acitretin in a 52-year-old man with HIV-associated psoriasis that responded poorly to previous treatments including steroids and ultraviolet B phototherapy. We also review the relevant literature.
Although some dermatologic diseases develop more frequently among people living with Human immunodeficiency virus (HIV) than among the general population, the prevalence of psoriasis is about the same in these groups at approximately 1-4% [1, 2]. When accompanied by severe pruritus and skin lesions, HIV-associated psoriasis may profoundly affect an individual's quality of life. Moreover, HIV-associated psoriasis responds poorly to treatment. Furthermore, treatment must be considered carefully because of the characteristics of immunosuppression and the immunosuppressive effect of systemic anti-psoriasis therapy [3]. Therefore, appropriate treatment is of great importance. So far, there has been no case report on HIV-associated psoriasis in the Korean literature despite extensive reviews in other languages. Here, we report a case of severe HIV-associated psoriasis successfully treated with acitretin and review the relevant literature.
A 52-year-old man living with HIV/AIDS was hospitalized because of severe pruritus with a generalized skin rash with scaling that began months ago and responded poorly to therapy with oral antihistamine and topical steroids. He was diagnosed with HIV-tuberculosis co-infection 4 years ago, with a CD4 count of 30 cells/mm3. Antiretroviral therapy was changed to boosted atazanavir and raltegravir owing to virologic failure. The subject had no history of smoking, alcohol consumption, lithium or β-blocker treatment, or family history of psoriasis.
Six months ago, he began visiting an outpatient clinic prompted by severe pruritus and rashes all over his body. Antihistamine and topical steroid ointment treatment had no effect. A skin biopsy was taken from a skin lesion on the posterior back, which was covered with scaly plaques. Optical microscopy revealed lymphocytes, macrophages, and multinucleated giant cells in corium-layer blood vessels. Irregular acanthosis, parakeratosis, and microabscesses were confirmed in the dead skin cells of the epidermal layer, which is consistent with psoriatic-form dermatitis (Fig. 1). The patient was treated with 3 courses of ultraviolet B radiation (300-397 mJ) delivered by a Cosmolux N-UVB 6,000 C (Choyang Medics, Seongnam, Korea).
The patient was admitted to the hospital because of aggravation of the skin lesions. On admission, blood pressure was 130/80 mmHg, pulse rate was 106 bpm, respiration rate was 20 breaths per minute, and body temperature was 36.6℃. Papuloerythematous skin lesions accompanied by silver-white scales were observed on his scalp and over his entire body (60% of body surface area) (Fig. 2A and B). No other unusual signs in the chest or abdominal area were observed. Neither arthralgia nor arthritis was observed in the extremities.
The laboratory test results were as follows: white blood cells, 4,800/mm3 (neutrophils, 68%; lymphocytes, 13.3%; monocytes, 9.7%; eosinophils, 8.2%); hemoglobin, 12.5 g/dL; platelets, 376,000/mm3; aspartate aminotransferase, 19 IU/L; alanine aminotransferase, 11 IU/L; alkaline phosphatase, 71 IU/L; total protein, 8.3 g/dL; albumin, 3.7 g/dL; total bilirubin, 0.4 mg/dL; blood urea nitrogen, 22 mg/dL; creatinine, 0.8 mg/dL; total cholesterol, 154 mg/dL; triglyceride, 181 mg/dL; and C-reactive protein, 0.26 mg/dL. Serology was negative for anti-hepatitis B virus and anti-hepatitis C virus antibodies. CD4 T-cell count was 44/mm3, and HIV RNA was 1.66 × 105 copies/mL.
Starting on the 1st hospitalization day, acitretin 30 mg (Neotigason™, Actavis Inc., Hafnarfjordur, Iceland) was prescribed to treat pruritus together with a topical steroid ointment. On the 8th day of acitretin administration, papuloerythematous skin lesions diminished and the scales disappeared (Fig. 2C and D). The dose of acitretin was reduced to 20 mg because of diminished pruritus. The patient was discharged on the 9th day.
When he visited an outpatient clinic 2 months later, most of the scales and papuloerythematous skin lesions had disappeared (Fig. 2E and F). Acitretin 10 mg was continued for 3 more months (Fig. 3). As of writing, the patient is still being monitored at the outpatient clinic and has not experienced relapse after 1.5 years.
Dermatologic disorders in people living with HIV/AIDS can be classified as primary or secondary. Primary diseases include seborrheic dermatitis, xerosis, atopic dermatitis, eosinophilic folliculitis, and psoriasis. Secondary diseases are associated with opportunistic viral, bacterial, or fungal infections and some neoplastic ones, which are more prevalent than in primary diseases [4]. Psoriasis causes severe pruritus and pain, and responds poorly to treatment; thus, it may profoundly affect an individual's quality of life and can even be fatal in some cases.
There is no agreed upon definition of HIV-associated psoriasis; the term is often used together with psoriasis in HIV seropositive patients out of confusion. Traits of HIV-associated psoriasis distinguishing it from classic seronegative psoriasis are sudden onset as well as its more severe, acral, extensive, and recalcitrant nature [4]. The disease also exhibits various morphological types in the same patients, appearing in one-third of their histories, along with a high frequency of arthritis. Notably, exacerbation due to staphylococcal and streptococcal infection is more common among HIV-infected individuals [5, 6]. In the present case, psoriasis began suddenly and was immediately severe (involving 60% of body surface area) during the course of diminished immune function (CD4 count, 44/mm3) without other risk factors such as excessive alcohol intake, smoking, and the use of drugs such as lithium and β-blockers [6]. No infectious complications occurred during treatment under close follow-up.
Psoriasis is associated with T-cell activation. Treatments that decrease T-cell count improve psoriasis, but HIV-associated psoriasis is more severe as a result of weakened immunosuppression [7, 8]; its risk is 9-fold greater with a CD4 count < 200/mm3 [9]. Thus, HIV-associated psoriasis acts as a marker of immune suppression. In addition, this makes HIV-associated psoriasis paradoxical. There are 3 competing hypotheses attempting to address this paradox. First, psoriasis is characterized by T cells that produce type-1 cytokines (i.e., interleukins 12 and 23, interferon γ, and tumor necrosis factor-α) [10]; however, in AIDS, T cells produce type-2 cytokines (i.e., interleukins 4, 5, and 10) [11]. The onset and subsequent exacerbation of psoriasis are associated with the numbers of CD8 T cells and their memory subsets in the dermis and epidermis of lesional skin [12, 13, 14]. CD8 T-cell counts are elevated in HIV-associated psoriasis, possibly accounting for the psoriasis [15]. The second hypothesis involves the depletion of CD4 suppressor T cells, which results in unchecked pro-inflammatory pathways due to the T cell imbalance, which may in turn cause psoriasis [16]. The third hypothesis states that because superantigen-mediated autoimmunity is characterized by autoreactive T cells bearing cutaneous lymphocyte-associated antigen, the recognition of autoantigens may result in molecular events that cause psoriasis [17, 18].
The treatment of HIV-associated psoriasis depends on the severity of the disease. Mild cases (< 2% body surface area) can be treated topically with emollients, corticosteroids, tar, vitamin D analogues, and retinoids. Meanwhile, moderate and severe (2-10% and < 10% of body surface area, respectively) cases can be treated with systemic therapies including phototherapy, acitretin, cyclosporin, hydroxyurea, and tumor necrosis factor-α inhibitors (i.e., etanercept or infliximab) with effective anti-retroviral treatments [6]. The treatment of moderate and severe HIV-associated psoriasis is challenging, and the risk-to-benefit ratio specific to these patients needs to be taken into account when selecting therapies.
In the present case of severe HIV-associated psoriasis, acitretin, a second-generation retinoid, was prescribed as a second-line treatment after the failure of first-line ultraviolet B phototherapy [19]. Buccheri et al. [20] report good to excellent responses in 6 (54%) of 11 patients with HIV-associated psoriasis and found complete clearance in 4 patients (36%) after 20 weeks of acitretin monotherapy.
Although oral retinoids are generally well tolerated, side effects can limit their use and negatively affect lipid profiles and liver functions [5]. HIV seropositive patients can have altered lipid profiles with hyperlipidemia at baseline. Accordingly, lipid profile and liver enzymes were closely monitored in the present case.
In summary, we report the first case of HIV-associated psoriasis in Korea. HIV-associated psoriasis is a serious condition that requires careful treatment selection and monitoring. This case report corroborates the safety and efficacy of acitretin therapy for the treatment of HIV-associated psoriasis. Nevertheless, additional case reports and research are required to clarify the characteristics and pathogenesis of psoriasis in patients with HIV.
Figures and Tables
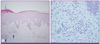 | Figure 1Pathologic findings. (A) The epidermis shows irregular acanthosis, partial parakeratosis, and focal microabscesses in the keratin layer (thin arrow). The upper dermis shows perivascular inflammatory cells infiltration (thick arrow) (hematoxylin & eosin, × 10). (B) High-power field, showing, some plasma cells (arrow), mature lymphocytes, and macrophages in the perivascular area (hematoxylin & eosin, × 40). |
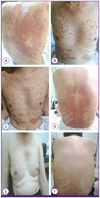 | Figure 2Photographs showing symmetrical, erythematous, and scaly plaques cutaneous lesion on the anterior trunk (A) and dorsum (B). On the 8th day of acitretin administration, the scaly lesions on the anterior trunk and dorsum had improved (C, D). After 2 months of treatment, the erythematous and scaly plaque cutaneous lesions nearly disappeared (E, F). |
 | Figure 3Treatments administered. Acitretin 30, 20, and 10 mg were administered for 21 weeks. Phototherapy involving 3 courses of ultraviolet B radiation (300-397 mJ) was administered for 6 weeks. Anti-retroviral therapy involving boosted atazanavir and raltegravir was administered throughout the treatment period. |
References
1. Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007; 370:263–271.

2. Muñoz-Pérez MA, Rodriguez-Pichardo A, Camacho F, Colmenero MA. Dermatological findings correlated with CD4 lymphocyte counts in a prospective 3 year study of 1161 patients with human immunodeficiency virus disease predominantly acquired through intravenous drug abuse. Br J Dermatol. 1998; 139:33–39.

3. Weitzul S, Duvic M. HIV-related psoriasis and Reiter's syndrome. Semin Cutan Med Surg. 1997; 16:213–218.

4. Cedeno-Laurent F, Gómez-Flores M, Mendez N, Ancer-Rodríguez J, Bryant JL, Gaspari AA, Trujillo JR. New insights into HIV-1-primary skin disorders. J Int AIDS Soc. 2011; 14:5.

6. Morar N, Willis-Owen SA, Maurer T, Bunker CB. HIV-associated psoriasis: pathogenesis, clinical features, and management. Lancet Infect Dis. 2010; 10:470–478.

7. Gupta AK, Ellis CN, Nickoloff BJ, Goldfarb MT, Ho VC, Rocher LL, Griffiths CE, Cooper KD, Voorhees JJ. Oral cyclosporine in the treatment of inflammatory and noninflammatory dermatoses. A clinical and immunopathologic analysis. Arch Dermatol. 1990; 126:339–350.

9. Goh BK, Chan RK, Sen P, Theng CT, Tan HH, Wu YJ, Paton NI. Spectrum of skin disorders in human immunodeficiency virus-infected patients in Singapore and the relationship to CD4 lymphocyte counts. Int J Dermatol. 2007; 46:695–699.

10. Lew W, Bowcock AM, Krueger JG. Psoriasis vulgaris: cutaneous lymphoid tissue supports T-cell activation and "Type 1" inflammatory gene expression. Trends Immunol. 2004; 25:295–305.

11. Klein SA, Dobmeyer JM, Dobmeyer TS, Pape M, Ottmann OG, Helm EB, Hoelzer D, Rossol R. Demonstration of the Th1 to Th2 cytokine shift during the course of HIV-1 infection using cytoplasmic cytokine detection on single cell level by flow cytometry. AIDS. 1997; 11:1111–1118.

12. Austin LM, Coven TR, Bhardwaj N, Steinman R, Krueger JG. Intraepidermal lymphocytes in psoriatic lesions are activated GMP-17(TIA-1)+CD8+CD3+ CTLs as determined by phenotypic analysis. J Cutan Pathol. 1998; 25:79–88.

13. Baker BS, Griffiths CE, Lambert S, Powles AV, Leonard JN, Valdimarsson H, Fry L. The effects of cyclosporin A on T lymphocyte and dendritic cell sub-populations in psoriasis. Br J Dermatol. 1987; 116:503–510.

14. Vissers WH, Arndtz CH, Muys L, Van Erp PE, de Jong EM, van de Kerkhof PC. Memory effector (CD45RO+) and cytotoxic (CD8+) T cells appear early in the margin zone of spreading psoriatic lesions in contrast to cells expressing natural killer receptors, which appear late. Br J Dermatol. 2004; 150:852–859.

15. Roederer M, Dubs JG, Anderson MT, Raju PA, Herzenberg LA, Herzenberg LA. CD8 naive T cell counts decrease progressively in HIV-infected adults. J Clin Invest. 1995; 95:2061–2066.

16. Fife DJ, Waller JM, Jeffes EW, Koo JY. Unraveling the paradoxes of HIV-associated psoriasis: a review of T-cell subsets and cytokine profiles. Dermatol Online J. 2007; 13:4.

17. Leung DY, Gately M, Trumble A, Ferguson-Darnell B, Schlievert PM, Picker LJ. Bacterial superantigens induce T cell expression of the skin-selective homing receptor, the cutaneous lymphocyte-associated antigen, via stimulation of interleukin 12 production. J Exp Med. 1995; 181:747–753.

18. Nickoloff BJ, Wrone-Smith T. Superantigens, autoantigens, and pathogenic T cells in psoriasis. J Invest Dermatol. 1998; 110:459–460.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download