Abstract
We report the case of a deep sternal wound infection with sternal osteomyelitis caused by Gordonia bronchialis after open-heart surgery. The isolate was identified as a G. bronchialis by 16S rRNA and hsp65 gene sequencing, having initially been misidentified as a Rhodococcus by a commercial phenotypic identification system.
Members of the genus Gordonia are aerobic, catalase-positive, Gram-positive, nonmotile, nocardioform actinomycetes that are weakly acid-fast [1]. Many Gordonia species has been isolated from soil [2] and Gordonia bronchialis has been detected in dogs [3], patients with sternal wounds [4], pleural fluid, tumor specimens, and skin [5, 6] among others.
As Gordonia species are closely related to members of the genera Nocardia and Rhodococcus, many clinical microbiology laboratories can misidentify Gordonia for Nocardia or Rhodococcus. Sternal wound infections, bacteremia, recurrent breast abscesses, and intraventricular shunt infections, all caused by G. bronchialis, have been reported [6]. This bacterium is difficult to identify to genus and species level because Gordonia species require extensive biochemical and morphological testing and an incubation time of at least 4 days for isolation [7]. Therefore, the use of broad-range polymerase chain reaction (PCR) and sequencing, with genes such as 16S rRNA or hsp65, is needed to identify the bacterium to species level [8]. Herein, we report a case of sternal osteomyelitis caused by G. bronchialis after open-heart surgery in a patient with diabetes mellitus.
A 69-year-old woman was admitted to hospital due to sternotomy site pain that had exacerbated 2 days prior. She had a 15-year history of hypertension and diabetes mellitus. The patient had undergone coronary artery bypass graft (CABG) surgery for triple vessel disease 2 months prior to presentation. She was discharged 9 days after the CABG surgery without complication. One week prior to the admission for the worsening sternotomy site pain, the patient presented in the outpatient clinic due to CABG operation site pain, but the pain was not relieved by opioids. Two days prior to admission, she developed redness, tenderness, and pus on the operation site, as well as wound dehiscence. At the time of admission, the patient was afebrile and complained of operation site pain with a slightly elevated C-reactive protein (CRP) level of 1.74 mg/dL. After obtaining specimens for ordinary bacterial, fungal, and mycobacterial culture, empirical therapy with vancomycin and cefotetan was initiated. The Gram stain showed many white blood cells, few epithelial cells, and only rare Gram-positive rods. Chest computed tomography on the second day of admission demonstrated cellulitis and focal osteomyelitis around the sternotomy site (Fig. 1), as well as focal fibrosis and linear atelectasis in the right-upper lobe, which were thought to be inflammatory sequelae. Her CRP level was normalized to 0.62 mg/dL on the sixth hospital day. On the eighth hospital day, wound closure was performed for wound dehiscence.
The wound culture on blood agar showed small, orange colored convex colonies without aerial hyphae after 5 days of incubation (Fig. 2). The organism was catalase-positive, oxidase-negative, and urease-positive gram-positive rod. It was preliminarily identified as an actinomycete, and as the treating physician believed the organism to be an anaerobic actinomycete, the antibiotics were changed to penicillin G for empirical therapy. The isolate was identified as a Rhodococcus species by a commercial identification system (API CORYNE, bioMérieux SA, Mercy l'Etoile, France) with the API code 1111004 (98% identification percentage), but the CAMP test was negative, demonstrating that the equi factor was not produced, and the Gram stain at 4 hours and 24 hours did not match the Rhodococcus species. The organism was identified as G. bronchialis DSM 43247T with a log score value of 1.808 by the MALDI-TOF mass spectrometry (Bruker Daltonics Inc., Billerica, MA, USA). A secondary match was indicated for Mycobacterium chlorophnolicum DSM 43826T with a log score value of 1.341. 16S rRNA gene sequencing of the organism was performed for further identification. PCR was performed according to previously published methods [9,10,11]. The purified PCR product was identified as G. bronchialis by 16S rRNA gene sequencing analysis, with 100% identity (1,324/1,324 bp) for G. bronchialis DSM 43247T (GenBank accession number CP001802). For secondary matches, the sequence of the isolate exhibited 98.6% (1,305/1,323) and 98.6% (1,304/1,322) identity for Gordonia rubripertincta DSM 43197T (GenBank accession number X80632) and Gordonia rhizosphera IFO 16247T (GenBank accession number AB023368), respectively (Fig. 3). In addition, the hsp65 sequence of the organism showed 99.8% (421/422) homology with the corresponding sequence of G. bronchialis strain DSM 43247T and 93.1% homology with that of Gordonia species KTR9 (GenBank accession number CP002907) [11]. In line with the Clinical and Laboratory Standards Institute (CLSI) guideline [12] the organism was identified as G. bronchialis. The patient was subsequently transitioned to imipenem administration. In follow-up wound culture on the 11th day, G. bronchialis was also grown after 5 days of incubation by pure culture. On the 18th day of admission, the patient had surgical wound debridement and curettage. A surgical specimen was drawn for modified acid-fast stain and Gram stain, which were negative, and a final wound culture was also negative.
An antibiotic susceptibility test was performed using the broth microdilution method, with a Sensititre RAPMYCO (Trek Diagnostic Systems, Part of Thermo Fisher Scientific, Oakwood Village, OH, USA) panel and interpreted using CLSI guideline M24-A2 [13]. The organism was susceptible to all antibiotics tested with minimum inhibitory concentrations (MICs) of trimethoprim/sulfamethoxazole ≤ 0.25/4.75 µg/mL, ciprofloxacin ≤ 0.12 µg/mL, moxifloxacin ≤ 0.25 µg/mL, amikacin ≤ 2 µg/mL, doxycycline ≤ 0.5 µg/mL, clarithromycin ≤ 2 µg/mL, linezolid ≤ 1 µg/mL, imipenem ≤ 2 µg/mL, cefepime ≤ 1 µg/mL, amoxicillin/clavulanate ≤ 2/1 µg/mL, ceftriaxone ≤ 4 µg/mL, minocycline ≤ 1 µg/mL, cefoxitin ≤ 8 µg/mL, tigecycline ≤ 0.5 µg/mL, and tobramycin ≤ 1 µg/mL.
For a few days before and then after undergoing CABG, the patient stayed in her daughter's house, where a dog was kept as a pet. We cultured specimens from the dog to determine the source of the organism, but these were all negative for G. bronchialis. After an 8 week course of intravenous imipenem, the wound healed completely and the patient was discharged symptom free.
We isolated G. bronchialis from wound cultures of a patient who had previously undergone CABG 2 months prior. G. bronchialis was the only microorganism cultured from the patient with infectious signs and the patient was the only patient with G. bronchialis infection in the hospital at the time. Therefore, it was a true pathogen. Sternal wound infection is a known manifestation of G. bronchialis infection [4, 14]. G. bronchialis has also been shown to cause a nosocomial outbreak of sternal wound infection. A seven patient outbreak of G. bronchialis sternal wound infections was traced to a nurse in 1991; the nurse's dog may have been the source of the outbreak strain isolated from the nurse [3]. Although we were unable to identify the source of infection in this case, the contact history of soil and pets was evaluated and any potential sources were cultured where possible.
Gordonia species infection is very rare but can cause systemic infections in immunocompromised patients [7, 15] and local infections in immunocompetent patients [16, 17]. A review of the previously reported cases of invasive Gordonia infection showed that more than 80% of cases were likely to have been catheter related [18]. Immunocompromise and the use of a catheter are factors that cause an increased likelihood of infections by Gordonia species.
As the number of recognized species of aerobic actinomycetes is rapidly increasing, assessment of the clinical significance of specific species is becoming increasingly important. Pathogen identification at the species level is closely related to pathogenicity, antimicrobial susceptibility, and the type of disease produced, and thus needs to be done accurately. Although Gordonia infection is clinically very important, it can be misidentified either because the isolate is considered to be an insignificant coryneform Gram-positive rod [18] or because it is mistakenly designated by routine biochemical tests as Nocardia or Rhodococcus [8]. Therefore, 16S rDNA or hsp65 gene sequencing is needed for identification of Gordonia species to the species level. Although MALDI-TOF mass spectrometry allows the rapid identification of aerobic actinomycetes from cultured colonies with a very short turnaround time, the isolate yielded log score between < 2 and ≥ 1.7 and thus could not be identified to species level.
A specific antibiotic treatment for G. bronchialis infection has not yet been standardized. Nonetheless, there is a precedent case in which ciprofloxacin and minocycline were used to treat G. bronchialis infection [6]. Moreover, another published report has recommended the use of carbapenem or a fluoroquinolone in combination with an aminoglycoside [18]. Therefore, as antibiotic treatment standards for Gordonia species are still somewhat controversial, the reporting of the MIC value as a pertinent threshold is still needed.
Penicillin, which was used for the patient in this report, had poor activity against Gordonia species, with 70% of tested isolates being susceptible [18]. The organism was preliminarily identified as an actinomycete so the physician believed the organism was an anaerobic actinomycete, and changed antibiotics to penicillin G for empirical therapy. After 16S rRNA and hsp65 gene sequencing, the organism was finally reported as G. bronchialis so the patient was subsequently transitioned to imipenem administration. Communication between physicians is important following preliminary identification of the organism.
To our knowledge, this is the first case of infection caused by G. bronchialis in a Korean subject. Arduous efforts to identify clinically significant pathogens are needed to enable appropriate treatment choices.
In conclusion, we report the case of G. bronchialis sternal osteomyelitis. In clinical laboratory testing, 16S rRNA or hsp65 gene sequencing was required to identify aerobic actinomycetes at the species level.
Figures and Tables
Figure 1
Chest computed tomography on the second day of admission demonstrated cellulitis and focal osteomyelitis around the sternotomy site (arrow).
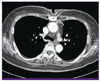
Figure 2
Colonies on blood agar showed small, orange colored convex colonies without aerial hyphae after 5 days of incubation (A) and large, wrinkled, orange colored colonies after 14 days of incubation (B). Gram stain of the pathogen from surgical wound tissue culture (×1,000, C).

Figure 3
Unrooted tree that showed the phylogenetic relationships of the current isolate (Case 5C467) and closely related Gordonia species.
The tree constructed using the neighbor-joining method was based on a comparison of 16S rRNA sequences. The phylogenetic tree was generated using ClustalW and visualized using TreeView. Distances are presented as number of substitutions per site (a scale of '0.1' means 0.1 nucleotide substitution per site).

References
1. Arenskötter M, Bröker D, Steinbüchel A. Biology of the metabolically diverse genus Gordonia. Appl Environ Microbiol. 2004; 70:3195–3204.

2. Shen FT, Goodfellow M, Jones AL, Chen YP, Arun AB, Lai WA, Rekha PD, Young CC. Gordonia soli sp. nov., a novel actinomycete isolated from soil. Int J Syst Evol Microbiol. 2006; 56:2597–2601.
3. Richet HM, Craven PC, Brown JM, Lasker BA, Cox CD, McNeil MM, Tice AD, Jarvis WR, Tablan OC. A cluster of Rhodococcus (Gordona) bronchialis sternal-wound infections after coronary-artery bypass surgery. N Engl J Med. 1991; 324:104–109.

4. Wright SN, Gerry JS, Busowski MT, Klochko AY, McNulty SG, Brown SA, Sieger BE, Ken Michaels P, Wallace MR. Gordonia bronchialis sternal wound infection in 3 patients following open heart surgery: intraoperative transmission from a healthcare worker. Infect Control Hosp Epidemiol. 2012; 33:1238–1241.

5. Aoyama K, Kang Y, Yazawa K, Gonoi T, Kamei K, Mikami Y. Characterization of clinical isolates of Gordonia species in Japanese clinical samples during 1998-2008. Mycopathologia. 2009; 168:175–183.

6. Johnson JA, Onderdonk AB, Cosimi LA, Yawetz S, Lasker BA, Bolcen SJ, Brown JM, Marty FM. Gordonia bronchialis bacteremia and pleural infection: case report and review of the literature. J Clin Microbiol. 2011; 49:1662–1666.

7. Drancourt M, Pelletier J, Cherif AA, Raoult D. Gordona terrae central nervous system infection in an immunocompetent patient. J Clin Microbiol. 1997; 35:379–382.

8. Gil-Sande E, Brun-Otero M, Campo-Cerecedo F, Esteban E, Aguilar L, García-de-Lomas J. Etiological misidentification by routine biochemical tests of bacteremia caused by Gordonia terrae infection in the course of an episode of acute cholecystitis. J Clin Microbiol. 2006; 44:2645–2647.

9. Bosshard PP, Abels S, Altwegg M, Böttger EC, Zbinden R. Comparison of conventional and molecular methods for identification of aerobic catalase-negative gram-positive cocci in the clinical laboratory. J Clin Microbiol. 2004; 42:2065–2073.

10. Relman DA. Universal bacterial 16S rRNA amplification and sequencing. In : Persing DH, Smith TF, Tenover FC, White TJ, editors. Diagnostic molecular microbiology principles and applications. 1st ed. Washington, DC: ASM Press;1993. p. 489–495.
11. Yin X, Liang S, Sun X, Luo S, Wang Z, Li R. Ocular nocardiosis: HSP65 gene sequencing for species identification of Nocardia spp. Am J Ophthalmol. 2007; 144:570–573.

12. Petti CA, Bosshard PP, Brandt ME, Clarridge JE, Feldblyum TV, Foxall P, Furtado MR, Pace N, Procop G. Interpretive criteria for identification of bacteria and fungi by DNA target sequencing : approved guideline. Wayne, PA: CLSI;2008.
13. Clinical and Laboratory Standards Institute. Susceptibility testing of mycobacteria, Nocardiae, and other aerobic actinomycetes; approved standard-second edition. M24-A2. Wayne, PA: CLSI;2011.
14. Tsukamura M. Proposal of a new genus, Gordona, for slightly acid-fast organisms occurring in sputa of patients with pulmonary disease and in soil. J Gen Microbiol. 1971; 68:15–26.

15. Drancourt M, McNeil MM, Brown JM, Lasker BA, Maurin M, Choux M, Raoult D. Brain abscess due to Gordona terrae in an immunocompromised child: case report and review of infections caused by G. terrae. Clin Infect Dis. 1994; 19:258–262.

16. Müller F, Schaal KP, von Graevenitz A, von Moos L, Woolcock JB, Wüst J, Yassin AF. Characterization of Rhodococcus equi-like bacterium isolated from a wound infection in a noncompromised host. J Clin Microbiol. 1988; 26:618–620.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download