Abstract
Although Mycobacterium avium complex (MAC) is the most common pathogen in nontuberculous mycobacterial (NTM) pulmonary diseases, endobronchial lesions caused by MAC infections are very rare even in an immunocompromised host. Herein, we describe the case of a 59-year-old, HIV-negative and non-immunocompromised woman who developed multifocal pulmonary infiltrations with endobronchial lesion caused by M. avium. Bronchoscopic examination revealed white- and yellow-colored irregular mucosal lesions in the bronchus of the left lingular division. M. avium was identified using sputum culture and bronchial washing fluid culture. Following the recommendations of the American Thoracic Society and Infectious Diseases Society of America (ATS/IDSA), the patient was begun on treatment with antimycobacterial drugs. After treatment, pneumonic infiltration decreased.
Nontuberculous mycobacteria (NTM) are widely distributed in the environment and can be found in the soil and in the water, including both natural and treated water sources. NTM may cause diseases in the lungs, lymph nodes, and skin, and have been shown to disseminate in severely immunocompromised patients. Pulmonary disease is the most common clinical manifestation of NTM [1]. In Korea, Mycobacterium avium complex (MAC), comprising M. avium and M. intracellulare, was the most frequently isolated pathogen in NTM pulmonary disease, followed by M. abscessus and M. kansasii [2]. Although pulmonary parenchymal disease due to NTM is a well-recognized phenomenon [1], endobronchial lesions as a result of NTM are extremely rare in either an immunocompetent or immunocompromised host. Thus far, very few cases of endobronchial NTM infection in an immunocompetent patient have been reported outside of Korea [3-8]. In Korean literature, there have been only two reports of immunocompetent patients with endobronchial disease caused by M. avium [9, 10]. Here, we report an additional case of endobronchial M. avium infection in an immunocompetent patient.
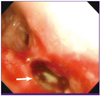
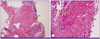
A 59-year-old woman was referred to the pulmonary department for evaluation of an abnormal finding detected on chest radiography and chest computed tomography (CT) during routine long-term follow-up for cancer. The patient had been diagnosed with cervical cancer and received surgery and adjuvant chemotherapy 10 years earlier. She had also undergone endoscopic mucosal resection for early stage rectal cancer two years ago. Since then, she has shown no evidence of recurrence of these cancers. Initially, during the routine follow-up, the patient displayed no abnormalities either in subjective symptoms or on physical examination. However, chest radiography and chest CT revealed multiple pulmonary infiltrates in the left lingular division and left lower lobe (Fig. 1A-1D). The complete blood cell count (CBC) and blood chemistry revealed the following: white blood cell (WBC) count of 4.87 × 103/mm3, with 69.8% neutrophils and 17.9% lymphocytes; platelet count of 283 × 103/mm3; hemoglobin level of 10.9 g/dL; aspartate transaminase level of 25 U/L; alanine transaminase level of 16 U/L; and total bilirubin level of 0.6 mg/dL. Anti-HIV antibody was negative. The patient was treated with empirically with antibiotics (amoxicillin/clavulanate), but did not show any interval changes on chest radiography. We recommended regular check-ups to monitor for any changes in the pulmonary lesions or other symptoms. Six months later, we performed follow-up chest radiography and chest CT. Although the patient still had no respiratory symptoms, chest images showed that the multiple pulmonary infiltrates had worsened (Fig. 2A-2D). We performed a bronchoscopic evaluation to obtain a differential diagnosis. Bronchoscopic examination revealed a white- and yellow-colored irregular mucosal lesion in the bronchus of the left lingular division (Fig. 3). Bronchoscopic biopsy of the endobronchial lesion showed chronic granulomatous inflammation with caseous necrosis, a typical and consistent feature of mycobacterial histopathology (Fig. 4A-4B). Acid-fast bacilli (AFB) staining was positive in bronchial washing fluid. However, the result of a real-time polymerase chain reaction (PCR) assay to detect M. tuberculosis was negative despite the use of a specimen that stained positive for AFB. Cytology for bronchial washing fluid was normal. Considering the slowly progressing nature of NTM pulmonary disease, we decided to postpone treatment, pending the results of AFB cultures. After two months, cultures of sputum and bronchial washing fluid grew NTM, and M. avium was identified in both of the specimens. In the meantime, the patient developed respiratory symptoms, including cough, sputum, and slight hemoptysis. Thus, antimycobacterial therapy was initiated with 500 mg of clarithromycin, 450 mg of rifampicin, and 800 mg of ethambutol daily. After two months of treatment, the patient no longer had respiratory symptoms and the infiltration of the left lower lobe on chest radiography had decreased. At time of this study, the patient was continuing to receive antimycobacterial therapy, without significant adverse reactions.
Although the incidence of pulmonary TB has been declining over the past several decades, the incidence of NTM pulmonary infection is increasing [11, 12]. NTM are ubiquitous organisms that are found in environmental reservoirs including water, soil, food, and animal carriers. Chronic pulmonary disease is the most common clinical manifestation of NTM infection. In Korea, MAC, M. abscessus, and M. kansasii are the most frequent NTM pulmonary pathogens, respectively [2]. Pulmonary involvement of NTM commonly occurs in patients with structural lung diseases such as chronic obstructive pulmonary disease, bronchiectasis, cystic fibrosis, pneumoconiosis, prior TB, pulmonary alveolar proteinosis, or esophageal motility disorder [1]. MAC pulmonary diseases typically present with abnormalities that take one of two forms on chest radiographs and chest CT scans. The first form is apical fibrocavitary lung disease in middle-aged men with a history of cigarette smoking, and the other is a bronchiectatic nodular form, frequently involving the right middle lobe or lingula, predominantly in postmenopausal nonsmoking women [1]. Our case is consistent with the bronchiectatic nodular form.
An endobronchial lesion due to NTM in an immunocompetent host is extremely rare. Up to this point, only eight such cases had been reported worldwide, including two cases in the Korean literature [3-10]. The first of the two reported Korean cases manifested as left main bronchus stenosis and atelectasis after that [9], and the second case took the multiple cavitary consolidation form, with rapid progression [10]. Unlike the previous two reported cases in Korea, our case was the typical bronchiectatic nodular form involving the lingular division, with insidious progression. A majority of these cases were caused by MAC and presented in various forms, which included actively caseating, edematous-hyperemic, fibrostenotic, tumorous, and granular, or a combination of these phenotypes. The findings of endobronchial lesions are similar to those of endobronchial TB [13, 14]. In the case presented here, bronchoscopic evaluation showed that the endobronchial lesion was due to M. avium and was an actively caseating form. Smart has suggested five potential mechanisms for the development of endobronchial infection due to M. tuberculosis: (1) direct extension from an adjacent parenchymal focus; (2) implantation of organisms from the infected sputum; (3) hematogenous dissemination; (4) lymph node erosion into the bronchus; and (5) through lymphatic drainage from parenchyma to the peribronchial region [14, 15]. The pathogenesis of endobronchial NTM remains unknown, but it might be similar to the pathogenesis of endobronchial TB. Shih et al. [3] have suggested, for example, that endobronchial lesions due to NTM might be caused by erosion of the mediastinal lymph node.
Diagnosis of M. avium pulmonary disease is generally difficult. Signs and symptoms are variable and nonspecific. Moreover, in our case, an accurate diagnosis was delayed for several months because symptoms only manifested themselves after the disease had advanced. During the follow-up period, the respiratory symptoms, including cough, sputum, and slight hemoptysis were presented. In addition, chest radiography and CT showed that previously identified pulmonary lesions progressed slowly. Bronchoscopic biopsy showed the presence of granulomatous inflammation with caseous necrosis, and the cultures from sputum and bronchial washing fluid grew M. avium. Finally, this patient was diagnosed with M. avium pulmonary disease according to ATS/IDSA criteria [1]. Although the patient had a previous history of cervical cancer and had been treated with chemotherapy, treatment had ceased 10 years earlier, and there had been no evidence of disease after that. Thus, this case corresponds to endobronchial NTM in an immunocompetent patient.
The diagnosis of NTM infection does not necessitate immediate treatment. The ultimate approach to NTM should be prudently decided upon, as the treatment requires a long duration, incurs a high cost, and is commonly associated with adverse reactions. According to ATS/IDSA guidelines [1], for most patients with nodular/bronchiectatic MAC disease, a regimen of 1000 mg of clarithromycin or 500-600 mg of azithromycin, 600 mg of rifampin, and 25 mg/kg of ethambutol 3 times a week is recommended. The recommended regimen for patients with fibrocavitary MAC disease or severe nodular/bronchiectatic disease is a daily regimen including 500-1000 mg of clarithromycin or 250-300 mg of azithromycin, 450-600 mg of rifampin, and 15 mg/kg of ethambutol. In addition, a parenteral drug (streptomycin or amikacin) is an option based on disease severity and treatment response. In the case presented here, considering the severe nodular/bronchiectatic form with an endobronchial lesion, we started the patient on a daily regimen of 500 mg of clarithromycin, 450 mg of rifampicin, and 800 mg of ethambutol.
In summary, we report a rare case of an endobronchial M. avium infection in an immunocompetent patient. Recently, the rate of NTM infection in the immunocompetent patient population has been increasing. Although the incidence of NTM disease is still rare, pulmonary infection caused by NTM should be considered in the differential diagnosis in patients who show an unfavorable response to the usual antibiotics or anti-TB medications.
Figures and Tables
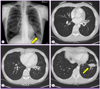 | Figure 1Chest radiography (A) and chest CT (B-D) at the initial visit showing multiple pulmonary infiltrates in the left lingular division and left lower lobe. |
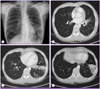 | Figure 2Chest radiography (A) and chest CT (B-D) after 6 months showing aggravation of the multiple pulmonary infiltrates in the left lingular division and left lower lobe. |
References
1. Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ Jr, Winthrop K. ATS Mycobacterial Diseases Subcommittee. American Thoracic Society. Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007. 175:367–416.

2. Koh WJ, Kwon OJ, Lee KS. Diagnosis and treatment of non-tuberculous mycobacterial pulmonary diseases: a Korean perspective. J Korean Med Sci. 2005. 20:913–925.

3. Shih JY, Wang HC, Chiang IP, Yang PC, Luh KT. Endobronchial lesions in a non-AIDS patient with disseminated Mycobacterium avium-intracellulare infection. Eur Respir J. 1997. 10:497–499.

4. Asano T, Itoh G, Itoh M. Disseminated Mycobacterium intracellulare infection in an HIV-negative, nonimmunosuppressed patient with multiple endobronchial polyps. Respiration. 2002. 69:175–177.

5. Fukuoka K, Nakano Y, Nakajima A, Hontsu S, Kimura H. Endobronchial lesions involved in Mycobacterium avium infection. Respir Med. 2003. 97:1261–1264.

6. Manali ED, Tomford WJ, Liao DW, Farver C, Mehta AC. Mycobacterium kansasii endobronchial ulcer in a nonimmunocompromised patient. Respiration. 2005. 72:305–308.

7. del Rio Camacho G, Soriano Guillén L, Flandes Aldeyturriaga J, Hernández García B, Bernácer Borja M. Endobronchial atypical mycobacteria in an immunocompetent child. Pediatr Pulmonol. 2010. 45:511–513.

8. Litman DA, Shah UK, Pawel BR. Isolated endobronchial atypical mycobacterium in a child: a case report and review of the literature. Int J Pediatr Otorhinolaryngol. 2000. 55:65–68.

9. Lee JH, Son KS, Park JH, Kim JC, Lee HW, Kim CH. Mycobacterium avium infection presenting as endobronchial lesions in an immunocompetent patient. Tuberc Respir Dis. 2006. 60:571–575.

10. Lee YJ, Kim DH, Yoon KH, Kim MY, Jung SW, Lee BK, Kim YJ. A case of pulmonary and endobronchial Mycobacterium avium infection presenting as an acute pneumonia in an immunocompetent patient. Tuberc Respir Dis. 2010. 69:279–283.

11. Park YS, Lee CH, Lee SM, Yang SC, Yoo CG, Kim YW, Han SK, Shim YS, Yim JJ. Rapid increase of non-tuberculous mycobacterial lung diseases at a tertiary referral hospital in South Korea. Int J Tuberc Lung Dis. 2010. 14:1069–1071.
12. Chen CY, Chen HY, Chou CH, Huang CT, Lai CC, Hsueh PR. Pulmonary infection caused by nontuberculous mycobacteria in a medical center in Taiwan, 2005-2008. Diagn Microbiol Infect Dis. 2012. 72:47–51.

13. Chung HS, Lee JH. Bronchoscopic assessment of the evolution of endobronchial tuberculosis. Chest. 2000. 117:385–392.

14. Kashyap S, Mohapatra PR, Saini V. Endobronchial tuberculosis. Indian J Chest Dis Allied Sci. 2003. 45:247–256.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download