Abstract
Emphysematous pyelonephritis (EPN) is a rare, life-threatening complication of upper urinary tract infections that is characterized by the presence of gas in the renal parenchyma and perirenal space. It commonly occurs in diabetic patients. Escherichia coli are the most common causative organisms, with few reports implicating Citrobacter freundii as the etiologic agent in EPN. A 57-year-old woman with diabetes and neurogenic bladder visited at our department with confused mentality, myalgia, and general weakness. Further investigation revealed that the patient suffered from unilateral EPN with sepsis caused by C. freundii. The patient's condition was improved considerably with percutaneous drainage and use of intravenous antibiotics for several weeks. However, renal function eventually deteriorated to permanent renal failure, which required hemodialysis. In conclusion, C. freundii may be the causative pathogen of EPN in a patient with type 2 diabetes and neurogenic bladder.
Emphysematous pyelonephritis (EPN), papillary necrosis, and perirenal abscess occur more frequently in people with diabetes and are associated with greater prevalence of bacteremia and acute deterioration of renal function [1-4]. Diabetes mellitus (DM) and ureteral obstruction can be predisposing factors leading to EPN [2, 5]. Complicated urinary tract infections (UTIs) are frequently caused by Escherichia coli and Klebsiella species [1-3, 6-9]. However, very few renal abscess or EPN cases have implicated Citrobacter species as the causative agent [10]. Only 3 cases of renal abscess or EPN caused by Citrobacter species have been reported in the past 40 years [10-12]. Citrobacter species, members of the aerobic Enterobacteriaceae family, are gram negative bacilli that are commonly found in the intestinal tract [13]. We herein report an unusual case of EPN caused by C. freundii in a type 2 diabetes patient.
A 57-year-old woman presented at the emergency department with confused mentality, fever, myalgia, and general weakness. The patient had a medical history of type 2 diabetes, hypertension, neurogenic bladder, and bilateral renal abscesses with sepsis caused by extended spectrum beta-lactamase (ESBL) producing E. coli, 1 year ago. Meropenem (1 g/day) was administered for approximately 4 weeks with an indwelling urinary catheter for approximately 2 weeks at that time.
Initial systemic examination revealed that the patient had a body temperature of 38.0℃, blood pressure of 138/64 mmHg, heart rate of 124 beats/min, respiratory rate of 34 breaths/min, tenderness in the left costovertebral angle, and peripheral pitting edema.
Further tests revealed that the patient had hypochromic normocytic anemia with hemoglobin levels of 9.3 g/dL, a white blood cell count of 13,700/mm3 (59% neutrophils and 26% band forms), and a platelet count of 194,000/mm3. Kidney function test revealed blood urea nitrogen (BUN) of 39.8 mg/dL and creatinine (Cr) of 3.9 mg/dL. Similar tests performed 1 year ago had revealed the BUN and Cr to be 31.9 mg/dL and 1.3 mg/dL, respectively. Furthermore, urinalysis revealed pyuria, glycosuria, and proteinuria without ketone bodies or nitrites. Arterial blood gas tests indicated that the pH of the patients' blood was 7.31, pCO2 were 35 mmHg, pO2 were 72 mmHg, SaO2 were 93%, and HCO3- were 17.6 mEq/L. Concentrations of C-reactive protein, sodium, potassium, and chloride were 11.12 mg/dL, 135 mEq/L, 6.7 mEq/L, and 102 mEq/L, respectively; tests for serum ketone body was negative. The initial blood glucose concentration was 411 mg/dL and glycosylated hemoglobin level was 9.8%. Concentrations of fibrin degradation product, fibrinogen, D-dimer, and anti-thrombin III were 26.8 µg/mL, 819 mg/dL, 1.5 µg/mL, and 82%, respectively. A chest radiograph showed bilateral pulmonary congestions.
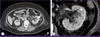
The patient further displayed clinical features of sepsis with renal failure in the form of fever, tachycardia, tachypnea, leukocytosis, azotemia with hyperkalemia, and pulmonary congestion. Initially, a broad-spectrum intravenous antibiotic (meropenem, 1 g/d) was administered based on prior admission history. Contrast-enhanced abdominal computed tomography (CT) images of the kidneys showed hydroureteronephrosis and a collection of gas bubbles with heterogenous enhancement in the enlarged left kidney (12.5 × 6 cm). Further, perinephric infiltration with fluid collection and wall thickening of the calyces, renal pelvis, and ureter were also observed (Fig. 1). Prompt percutaneous nephrostomy (PCN) with a pig-tail tube was performed in the left kidney, resulting in 50 mL of pus being drained with air. Microbiological examination of both blood and pus obtained from the PCN revealed the presence of C. freundii. As a result, the antibiotic regimen was switched to a third generation cephalosporin based on the results of culture and sensitivity tests. The patient responded to the treatment and became afebrile and clinically stable. PCN was maintained for 2 weeks and the antibiotic was continued for 6 weeks. After treatment, an abdominal CT showed improved lesions of left kidney with no gas bubbles detected in the renal parenchyma, pelvis, and calyces. Despite the improvements in patient's condition following treatment, the patient developed permanent kidney failure, which required hemodialysis.
Diabetes mellitus is the single most common disorder associated with EPN. Up to 95% of patients with EPN have underlying uncontrolled diabetes mellitus [7, 13]. Other reported underlying factors associated with the development of EPN are drug abuse, neurogenic bladder, alcoholism, and anatomic anomaly [1, 14, 15]. A high number of patients with urinary tract obstruction develop EPN as a secondary complication (25.40%) [1, 14].
A recent study indicated that E. coli (61.9%), Candida species (12.7%), and gram-positive cocci (12.7%) were the common microorganisms isolated from the urine cultures of patients with diabetes and a clinical diagnosis of UTI [9]. Furthermore, E. coli (69%) and Klebsiella pneumonia (29%) were reported to be the most common pathogens in diabetic patients with EPN [1, 3, 6-8]. Interestingly, in rare cases, anaerobic microorganisms including Clostridium septicum, Candida albicans, Cryptococcus neoformans, and Pneumocystis jiroveci have been isolated from diabetic patients with EPN [2].
In this study, we detected C. freundii in a diabetic patient who developed EPN. Citrobacter infections typically occur in hospital settings in patients with multiple co-morbidities. In the majority of cases, patients are infected with Citobacter koseri and/or C. freundii [10, 15]. In most cases of infection with Citrobacter species, a UTI (41 out of 78, 52.5%) was the most common clinical diagnosis; further, 30 of these patients had acute pyelonephritis, 3 had acute cystitis, and 8 had bacteriuria. Citrobacter infections (C. koseri, 90.2%; C. freundii, 9.8%) are generally described as uncomplicated UTIs (46.2%) [14]. Furthermore, renal abscess have been associated with an ascending infection and rarely with Citrobacter species.
Three cases of renal abscess or EPN caused by a Citrobacter infection have been reported. In the first study, Citrobacter perinephric abscess were detected in a kidney transplant recipient [11]. In 1996, another instance of EPN caused by Citrobacter was detected in a diabetic patient with tuberculous kidney disease [12]. A high-dose antibiotic regimen was administered, but a nephrectomy was necessary to avert a life-threatening situation. Finally, gas-forming renal and hepatic abscess were detected and found to be attributed to C. koseri infection in a patient with type 2 diabetes suffering from liver cirrhosis [10].
Even though, Citrobacter species rarely cause emphysematous infections, poor glycemic control that might accelerate the fermentation of glucose leading to gas production. Furthermore, the functional obstruction of a neurogenic bladder may decrease the clearance of gas.
Percutaneous drainage or emergency nephrectomy with appropriate antibiotics may be required, although treatment guidelines for EPN are still not established [1-4]. Khaira et al. concluded that the availability of renal support reduces the mortality rate [13]. The long term outcome for renal function will depend on the degree of parenchymal loss and the coexisting renal disease. In this case, the C. freundii infection was controlled with renal support in the form of hemodialysis; however, renal function deteriorated over time probably due to underlying diabetic nephropathy.
Figures and Tables
References
1. Huang JJ, Tseng CC. Emphysematous pyelonephritis: clinicoradiological classification, management, prognosis, and pathogenesis. Arch Intern Med. 2000; 160:797–805.
4. Abdul-Halim H, Kehinde EO, Abdeen S, Lashin I, Al-Hunayan AA, Al-Awadi KA. Severe emphysematous pyelonephritis in diabetic patients: diagnosis and aspects of surgical management. Urol Int. 2005; 75:123–128.

5. Patterson JE, Andriole VT. Bacterial urinary tract infections in diabetes. Infect Dis Clin North Am. 1997; 11:735–750.

6. Bjurlin MA, Hurley SD, Kim DY, Cohn MR, Jordan MD, Kim R, Divakaruni N, Hollowell CM. Clinical outcomes of nonoperative management in emphysematous urinary tract infections. Urology. 2012; 79:1281–1285.

7. Dubey IB, Agrawal V, Jain BK. Five patients with emphysematous pyelonephritis. Iran J Kidney Dis. 2011; 5:204–206.
8. Farrell DJ, Morrissey I, De Rubeis D, Robbins M, Felmingham D. A UK multicentre study of the antimicrobial susceptibility of bacterial pathogens causing urinary tract infection. J Infect. 2003; 46:94–100.

9. Kofteridis DP, Papadimitraki E, Mantadakis E, Maraki S, Papadakis JA, Tzifa G, Samonis G. Effect of diabetes mellitus on the clinical and microbiological features of hospitalized elderly patients with acute pyelonephritis. J Am Geriatr Soc. 2009; 57:2125–2128.

10. Lin SY, Ho MW, Yang YF, Liu JH, Wang IK, Lin SH, Huang CC. Abscess caused by Citrobacter koseri infection: three case reports and a literature review. Intern Med. 2011; 50:1333–1337.

11. Williams RD, Simmons RL. Citrobacter perinephric abscess presenting as pneumoscrotum in transplant recipient. Urology. 1974; 3:478–480.

12. Fischer C, Kallerhoff M, Weidner W, Ringert RH. Citrobacter emphysematous pyelonephritis in a tuberculous kidney caused by citrobacter A case report in a diabetic patient. Ann Urol (Paris). 1996; 30:108–111.
13. Khaira A, Gupta A, Rana DS, Gupta A, Bhalla A, Khullar D. Retrospective analysis of clinical profile prognostic factors and outcomes of 19 patients of emphysematous pyelonephritis. Int Urol Nephrol. 2009; 41:959–966.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download