Abstract
Background
Materials and Methods
Results
Conclusions
Figures and Tables
 | Figure 1Time and frequency of causative organisms (n = 135) isolated in bloodstream infections after liver transplantation.
GPC, gram-positive cocci; GNB, gram-negative bacilli.
|
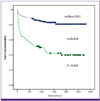 | Figure 2
Effect of bloodstream infection in the year after transplantation on Kaplan-Meier survival curves of liver transplant recipients. BSI, bloodstream infection. |
Table 1

SD, standard deviation; HCC, hepatocellular carcinoma; IQR, interquartile range; MELD, model for end stage liver disease.
aMay have > 1 underlying liver disease.
bA few cases were proven to have small HCC at postoperative examination.
cIncludes toxic hepatitis (n = 3) and A-viral hepatitis (n = 2).
dIncludes cholangiocarcinoma (n = 3), angiosarcoma (n = 1), and hemangioendothelioma (n = 1).
eIncludes Budd-Chiari syndrome (n = 2), hemophilia (n = 1), and Wilson disease (n = 1).
fUse of broad-spectrum antibiotics for > 5 days in the month before liver transplantation.
gScore indicates the severity of liver disease, and ranges from 5 to 15 according to the degree of as cites, the serum concentrations of bilirubin and albumin, the prothrombin time, and the degree of encephalopathy.
hScore indicates hepatic dysfunction, and ranges from 6 to 40 or more according to the following formula: MELD = 3.8 [Ln serum bilirubin (mg/dL)] + 11.2 [Ln INR] + 9.6 [Ln serum creatinine (mg/dL)] + 6.4.
Table 3
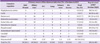
BSI, bloodstream infection; IQR, interquartile range; Abd/Wound, abdominal or wound infection; IVC, intravascular catheter infection; CoNS, coagulase-negative staphylococci.
aMedian time in days from the date of liver transplantation to the onset of bloodstream infection.
bIncludes P. aeruginosa (10), P. fluoroscens (1), P. putida (1).
cIncludes C. albicans (9), C. tropicalis (5), C. krusei (2), C. glabrata (1), C. parapsilosis (1), C. lusitaniae (1).
dIncludes Serratia marcescens (1 from abdomen), Pantoea species (1 from abdomen), and Stenotrophomonas maltophilia (1 from abdomen and the other from lung).
eShows isolated organisms in episodes of polymicrobial BSI/total BSI stratified by its origin. Each isolate was counted as a single case, which resulted in 135 organisms from 112 BSI episodes.
Table 4

Continuous data normally distributed are expressed as mean (SD) and analyzed using students t-test. All other continuous data not normally distributed are presented as median (IQR) and analyzed using Mann-Whitney U-test.
BSI, bloodstream infection; IQR, interquartile range; MELD, model for end stage liver disease; pRBC, packed red blood cell; ICU, intensive care unit; LT, liver transplantation.
aBinary logistic regression model was used for univariate analysis.
bMay have > 1 underling liver disease.
cUse of broad-spectrum antibiotics for > 5 days in 1 month before liver transplantation.
dIncludes biliary leakage (n = 20), biliary stricture (n = 42), and other overlapping biliary complications (n = 10).
Table 5

BSI, bloodstream infection; OR, odds ratio; CI, confidence interval; ICU, intensive care unit; LT, liver transplantation; IQR, interquartile range.
aBinary logistic regression model was used for multivariate analysis. Candidate risk factors in Table 5 were all entered and only significant parameters are listed.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download