Abstract
Background
The number of cases of pertussis reported has increased gradually in the last decade. Pertussis vaccination is the most effective strategy for the prevention of infection. Despite the fact that young infants are at the highest risk for pertussis, the rate of tetanus-diphtheria-acellular pertussis (Tdap) vaccination is presumed to be very low among women of childbearing age in Korea. The purpose of this study was to investigate the perceptions of women of childbearing age regarding Tdap vaccination in Korea.
Materials and Methods
Women of childbearing age, who visited the Department of Obstetrics and Gynecology at 3 University hospitals in the Seoul and Gyeonggi-do provinces of Korea, were surveyed. Individual questionnaires were administered from April to May 2012. Demographic data, Tdap vaccination history, general knowledge about pertussis, and information on factors associated with decision on vaccination were collected.
Results
Of the 500 reproductive-age women enrolled, only 4 (0.8%) had received the Tdap. The most common reason for non-vaccination was the lack of awareness of pertussis and information about the Tdap. Totally, 171 (34.2%) responded that they would receive a Tdap vaccination in the future. By multivariate analysis, general confidence in the effectiveness of the vaccine (odds ratio [OR] = 1.88, 95% confidence interval [CI] 1.17 to 3.01) was indicated as an important factor for deciding whether to receive the Tdap vaccine (P < 0.01).
Conclusions
The coverage of Tdap vaccination of women of childbearing age, including pregnant women, is very low because of the lack of awareness of pertussis and the Tdap. Education of women of childbearing age about pertussis is very important to increase Tdap vaccination rates among these women, particularly during the perinatal period.
Pertussis, caused by Bordetella pertussis, is an acute, highly infectious disease. Despite high vaccination rates among children, an increase in the incidence of pertussis has been reported in recent years in some countries [1-4]. Pertussis remains a serious potential health risk to infants. Most deaths occur in the first 6 months of life, in those too young to be vaccinated or those who did not a complete vaccination schedule [5]. Adolescents and adults who come in close contact with young infants are the primary source of infection to susceptible and unprotected infants [6]. The primary goal of Tdap vaccination is to protect infants from severe disease. Vaccination of pregnant women and those in close contact with infants is recommended to protect very young infants. The latest Advisory Committee on Immunization Practice (ACIP) recommendation is to administer the Tdap to all unvaccinated pregnant women and adolescents and adults who come in close contact with infants aged < 12 months [7]. In Korea, the Tdap vaccination is not currently recommended during pregnancy; persons aged 11-64 years and postpartum women who have frequent contact with infants aged < 1 year are the main target groups for Tdap vaccination [8]. The recent estimated vaccination coverage of DTaP in a timely manner (at least 4 times) among Korean children ranged between 65.7% and 94.0%, but there is no such concrete data on the Td or Tdap coverage for adults [9] as well as women of childbearing age. Therefore, we studied the Tdap coverage rate and factors influencing the personal decision to receive Tdap among women of childbearing age in Korea.
From April to May 2012, 20 to 49 year-old women who visited the Department of Obstetrics and Gynecology at 3 university hospitals located in Seoul and Gyeonggi-do Province and volunteered to participate our research were enrolled. The study population also included women receiving antenatal or postnatal care. We planned to enroll more than 496 women (level of significance, 0.05; power, 0.8), because previous studies reported a significant 5-12% rate of maternal influenza vaccination [10-13].
Trained interviewers at each institution conducted individual interviews using a questionnaire. The questionnaire was composed of questions on demographic characteristics, socioeconomic indicators, awareness of the efficacy and safety of vaccines, previous Tdap vaccination history, factors associated with the decision of receiving the vaccine, and other factors associated with Tdap vaccination plans. Knowledge about pertussis was rated on a scale of 0 to 3 points according to answers to the 3 questions focused on pertussis.
The demographics and clinical characters of respondents were descripted using descriptive statistics. The differences in general perceptions on vaccination were analyzed using The Chi-square test. Tdap vaccination-related factors were analyzed using The Chi-square test and Fisher's exact test. To find the factors that influence whether receive the Tdap, multiple logistic regression was performed. Used factors were previous history of childbirth, education, income, number of underlying disease, positive thinking on effectiveness of the vaccine, concern on the vaccine safety, perception on pertussis, awareness of Tdap, recommendation from obstericians or gynecologists. The statistical program SPSS, version 17.0 (IBM Corporation, Armonk, NY, USA), was used for these analyses. A P-value of < 0.05 (two-tailed) was used to establish statistical significance.
A total of 500 women of reproductive age responded to the survey. The median age of the respondents was 32 years. Of these, 107 (21.4%) women were pregnant, and their median gestational age was 24 (IQR 19) weeks (Table 1). Among the survey respondents, the number of women who previously had at least one pregnancy was 236 (47.2%). Of all the respondents, 235 (47.0%) had experienced more than one live birth; in addition, the number of women who had more than one child was 129 (25.8%), and the number of women who had only one child was 106 (21.2%). The average age of the first child was 7 (IQR 8) years. Of the 500 respondents, 372 (74.4%) held a bachelor's degree or higher level of education, and the average monthly income of the family was more than 3 million won (n = 334; 66.7%). Sixty-eight (14.6%) respondents had more than one chronic disease, and thyroid disease was the most common chronic disease (n = 27; 39.7%).
Three hundred eighteen (63.6%) respondents had a generally positive perception toward the effectiveness of the vaccine, while 307 (61.4%) had concerns about its adverse reactions. Of the 500 respondents, 344 (68.8%) were vaguely aware of the existence of pertussis, and 78 (15.6%) were well informed-about it. Sixty-four (12.8%) of total 500 women were aware of the necessity of Tdap vaccination for adults, but only 20 (4.0%) of the respondents were recommended to receive the Tdap by their obstetrician or gynecologist.
Among all the respondents, only 4 (0.08%) had received the Tdap (Table 2). The most common reason for not receiving the Tdap was the lack of knowledge on pertussis (n = 276; 55.8%). The most important factor for non-vaccination was the lack of recognition of the existence or necessity of the Tdap for adults (n = 268; 54.1%). The second most important factor was the lack of recommendation by a healthcare provider (n = 115; 23.2%).
Out of a total of 500 respondents, 171 (34.2%), including 33 pregnant women, 16 breastfeeding women, and 122 women of childbearing age (excluding pregnant or breastfeeding women), answered that they would consider receiving the Tdap in the future. Among the 122 women who were not pregnant or lactating, 59 (47.2%) decided to receive the Tdap because they were aware of the risk of pertussis and 39 (31.2%) respondents intended to receive the Tdap if their healthcare provider recommended them to do so (Table 3). Among the women who refused the receiving the Tdap in the future, the leading causes for rejection were inadequate knowledge about the severity of pertussis (n = 130; 54.6%) and insufficient information about the necessity of the Tdap (n = 146; 61.3%).
For pregnant women, the factors associated with vaccination and non-vaccination of Tdap were the same. Of 107 pregnant women, 33 (30.8%) said that they would receive the vaccination and 17 (51.5%) said that they would receive the Tdap on their healthcare provider's recommendation (Table 4). The most common reason for non-vaccination among pregnant women was the lack of knowledge about pertussis (n = 48, 65.8%).
In the case of breastfeeding women, 16 (50.0%) of 32 breastfeeding women indicated that they would receive the Tdap (Table 5). The most common reason for deciding to receive the Tdap in the future was the recommendation of a healthcare provider (n = 8, 50.0%), which was also the reason why pregnant women received the vaccination. In contrast, the most common reason for not receiving the Tdap was concern about its harmful effects on a breastfeeding child (n = 8, 50.0%).
In the univariate analysis, factors associated with the decision to receive the Tdap in the future were a positive thinking toward the ectiveness of vaccination (P < 0.01), knowledge of pertussis (P = 0.02), and awareness of the necessity of Tdap vaccination for adults (P < 0.01) (Table 6). Used factors were previous history of childbirth, education, income, number of underlying disease, positive thinking on effectiveness of the vaccine, concern on the vaccine safety, perception on pertussis, awareness of Tdap, recommendation from obstericians or gynecologists. In the multivariate analysis, statistically significant factors associated with the intention to receive Tdap in the future were a positive attitude toward the effectiveness of vaccination (OR: 1.80; 95% CI: 1.20 to 2.72, P<0.01) and awareness of the necessity of Tdap vaccination for adults (OR: 3.36, 95% CI: 1.94 to 5.08, P < 0.01).
Out of a total of 500 respondents, 238 (47.6%) women answered that they would recommend the Tdap vaccination for their family.
Of the women of childbearing age, those who had previously received Tdap accounted for only 0.08% (n = 4) of all responders in this study, in spite of the recent substantial rise in the cases of pertussis reported in Korea.
The majority of pertussis cases, hospitalizations, and deaths occur in infants aged ≤ 2 months of age, who are too young to be vaccinated [7]. In Korea, the most common age groups affected by pertussis during 2010-2011 were those aged < 3 months and ≥ 15 years; intrafamilial transmission was common among these groups [9]. Considering that infants aged < 3 months usually stay at home and have no interaction with others besides their immediate family members, conducting a sensitive and timely examination of the asymptomatic or atypical symptomatic family members should be a public health priority [14]. Pertussis vaccination is the most effective strategy to reduce morbidity and mortality due to pertussis [15]. Therefore, the cocooning strategy, which involves immunizing all household and key persons in close contact with the newborns, with the Tdap, is suggested [16]. Usually, mothers are the key persons in contact with newborns, and therefore, Tdap vaccination for all women of childbearing age is very important for reducing morbidity and mortality due to pertussis among infants aged < 3 months.
In infants, maternal vaccination can prevent infections acquired during delivery through the placenta, until immunity is induced by active immunization. The Tdap should be administered to all pregnant woman on the basis of an assessment of its benefits versus its risks [17-19]. Several studies on the direct protection of infants by neonatal monovalent acellular pertussis vaccination reported that the vaccination could significantly increase the level of immunoglobulin G (IgG) antibody against pertussis antigen, but it also could result in interference in the development of immunity against hepatitis B and Haemophilus influenza type B [20-22]. Therefore, the current ACIP recommendation is to administer the Tdap to unvaccinated pregnant women in the third or late second (after 20 weeks gestation) trimester [7]. In Korea, there isn't any pertussis outbreak in these days. It is recommended that previously unvaccinated women of childbearing age receive the Tdap before pregnancy and postpartum Tdap vaccination is recommended for previously unvaccinated pregnant women. In the case of a pertussis outbreak, it is recommended that unvaccinated pregnant women receive the Tdap. Additionally, Tdap vaccination is recommended for all adults who are in frequent contact with infants aged < 1 year and family members who have neonates [8].
Despite the very low Tdap vaccination rate (0.04%) among women of childbearing age, all women who had received the vaccine had been advised to receive it by their healthcare provider, and 2 women (50.0%) were advised by their obstetrician or gynecologist. A recommendation from the healthcare provider was the leading factor influencing women of childbearing age to receive the Tdap vaccination. However, only 20 of the 500 women of childbearing age who visited the Department of Obstetrics and Gynecology received a recommendation for Tdap vaccination from their obstetrician or gynecologist. Education and a recommendation from an obstetrician or gynecologist about Tdap vaccination for women of childbearing age are necessary before and after pregnancy. The most common and important reason for not receiving the Tdap was the lack of information about the Tdap. Thus, it is important for the obstetrician or gynecologist to provide information about the necessity of receiving a Tdap and to recommend it actively.
One hundred seventy-one (34.2%) women replied that they would receive the Tdap in the future. The perception on risk of pertussis infection (n = 75, 43.9%) and a recommendation from a healthcare provider (n = 64, 37.4%) were the most common factors for receipt of the Tdap. In contrast, 329 (65.8%) respondents answered that they did not plan to receive the vaccination in the future, because they were unaware of pertussis and the Tdap vaccination (n = 276; 55.8%). The majority of women did not know that pertussis was a vaccine-preventable disease, although they knew that it was an infectious disease. It is possible that women might not feel the need to be vaccinated because they do not recognize the risk of pertussis infection. In fact, in the multivariate analysis of factors associated with Tdap vaccination plans, the statistically significant factors were general reliance on knowledge about vaccine effectiveness (OR: 1.80, 95% CI: 1.20 to 2.72) and awareness of the necessity of Tdap adults (OR: 3.36, 95% CI: 1.94 to 5.08). Concern about the adverse reaction of the vaccine was not an important factor for the decision of receiving the Tdap. Therefore, as a factor affecting the decision to receive the Tdap, gaining knowledge about the Tdap was more significant than having very little knowledge about pertussis.
The results of this study suggest that the major obstacles to increase the rate of Tdap vaccination among women of childbearing age are both unawareness about pertussis and Tdap and the lack of recommendation from a healthcare provider. Of the 500 respondents, 171 (34.4%) women decided to receive the Tdap. A recent study conducted to assess the decision-making process of women who accepted or declined receiving postpartum pertussis vaccination revealed 53% acceptance of vaccination among Taiwanese women [23]. To improve the Tdap vaccination rate among women of childbearing age, it is important that healthcare providers, including obstetricians and gynecologists, thoroughly brief women of childbearing age about the disease burden of pertussis for infants and actively recommend the Tdap.
This study had several limitations. First, the participants did not represent the entire population of women of childbearing age, as only those who visited the university hospital were recruited. Second, vaccination histories could be inaccurate because they were based on self-reporting without any confirmation from medical records.
In conclusion, the rate of Tdap vaccination among women of childbearing age is very low in Korea. To improve this vaccination rate, a recommendation from a healthcare provider and education of pertussis and the Tdap are important.
Figures and Tables
Table 1
Baseline characteristics of the respondents
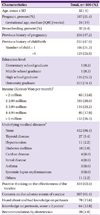
aOne respondent did not answer this question.
bSome respondents had one or more underlying medical diseases.
cOne point for each question out of 3 questions about knowledge on pertussis.
Only respondents who have heard about pertussis and had knowledge about it answered the questions (Supplementary 1).
Table 2
Previous Tdap vaccination history and reasons for vaccination of women of childbearing age in Korea

Table 3
Future plans for Tdap vaccination and reasons for vaccination (women of childbearing age excluding pregnant and lactating women)

References
1. Centers for Disease Control and Prevention (CDC). Pertussis--United States, 2001-2003. MMWR Morb Mortal Wkly Rep. 2005. 54:1283–1286.
2. Tsuji Y. Current trend and importance of prevention on pertussis. Nihon Rinsho. 2009. 67:2207–2212.
3. Campbell H, Amirthalingam G, Andrews N, Fry NK, George RC, Harrison TG, Miller E. Accelerating control of pertussis in England and Wales. Emerg Infect Dis. 2012. 18:38–47.

4. Guiso N, Wirsing von König CH, Forsyth K, Tan T, Plotkin SA. The global pertussis initiative: report from a round table meeting to discuss the epidemiology and detection of pertussis, Paris, France, 11-12 January 2010. Vaccine. 2011. 29:1115–1121.

5. Vitek CR, Pascual FB, Baughman AL, Murphy TV. Increase in deaths from pertussis among young infants in the United States in the 1990s. Pediatr Infect Dis J. 2003. 22:628–634.

6. Bechini A, Tiscione E, Boccalini S, Levi M, Bonanni P. Acellular pertussis vaccine use in risk groups (adolescents, pregnant women, newborns and health care workers): a review of evidences and recommendations. Vaccine. 2012. 30:5179–5190.

7. Centers for Disease Control and Prevention (CDC). Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine (Tdap) in pregnant women and persons who have or anticipate having close contact with an infant aged <12 months --- Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011. 60:1424–1426.
8. Cheong HJ. Vaccination for adult. 2012. 2nd ed. Seoul: MIP;133–151.
9. Choe YJ, Park YJ, Jung C, Bae GR, Lee DH. National pertussis surveillance in South Korea 1955-2011: epidemiological and clinical trends. Int J Infect Dis. 2012. 16:e850–e854.

10. Fisher BM, Scott J, Hart J, Winn VD, Gibbs RS, Lynch AM. Behaviors and perceptions regarding seasonal and H1N1 influenza vaccination during pregnancy. Am J Obstet Gynecol. 2011. 204:6 Suppl 1. S107–S111.

11. Goldfarb I, Panda B, Wylie B, Riley L. Uptake of influenza vaccine in pregnant women during the 2009 H1N1 influenza pandemic. Am J Obstet Gynecol. 2011. 204:6 Suppl 1. S112–S115.

12. Naleway AL, Smith WJ, Mullooly JP. Delivering influenza vaccine to pregnant women. Epidemiol Rev. 2006. 28:47–53.

13. Kim MJ, Lee SY, Lee KS, Kim A, Son D, Chung MH, Park SG, Park JH, Lee BI, Lee JS. Influenza vaccine coverage rate and related factors on pregnant women. Infect Chemother. 2009. 41:349–354.

14. Shakib JH, Wyman L, Gesteland PH, Staes CJ, Bennion DW, Byington CL. Should the pertussis case definition for public health reporting be refined? J Public Health Manag Pract. 2009. 15:479–484.

15. Centers for Disease Control and Prevention (CDC). Pertussis epidemic--Washington, 2012. MMWR Morb Mortal Wkly Rep. 2012. 61:517–522.
16. Castagnini LA, Healy CM, Rench MA, Wootton SH, Munoz FM, Baker CJ. Impact of maternal postpartum tetanus and diphtheria toxoids and acellular pertussis immunization on infant pertussis infection. Clin Infect Dis. 2012. 54:78–84.

17. Gall SA, Myers J, Pichichero M. Maternal immunization with tetanus-diphtheria-pertussis vaccine: effect on maternal and neonatal serum antibody levels. Am J Obstet Gynecol. 2011. 204:334.e1–334.e5.

18. Leuridan E, Hens N, Peeters N, de Witte L, Van der Meeren O, Van Damme P. Effect of a prepregnancy pertussis booster dose on maternal antibody titers in young infants. Pediatr Infect Dis J. 2011. 30:608–610.

19. Mooi FR, de Greeff SC. The case for maternal vaccination against pertussis. Lancet Infect Dis. 2007. 7:614–624.

20. Knuf M, Schmitt HJ, Jacquet JM, Collard A, Kieninger D, Meyer CU, Siegrist CA, Zepp F. Booster vaccination after neonatal priming with acellular pertussis vaccine. J Pediatr. 2010. 156:675–678.

21. Knuf M, Schmitt HJ, Wolter J, Schuerman L, Jacquet JM, Kieninger D, Siegrist CA, Zepp F. Neonatal vaccination with an acellular pertussis vaccine accelerates the acquisition of pertussis antibodies in infants. J Pediatr. 2008. 152:655–660. 660.e1

Supplementary material
Supplementary data regarding one questionnaire of survey can be found with this article online http://www.icjournal.org/src/sm/ic-45-217-s001.pdf.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download