Abstract
Background
The cases of Plasmodium vivax malaria in Korea are mixed with long and short incubation periods. This study aims to define clinico-epidemiologic chracteristcs of Plasmodium vivax malaria in Korea.
Materials and Methods
We selected the civilian cases infected with P. vivax malaria in Korea from the epidemiological investigation data of 2001 to 2010, whose incubation periods could be estimated. The long and short incubation periods were defined by duration of infection and onset time, and the cases were compared by demographic factors and clinical symptom, infection and onset time. The correlation was analyzed between the proportion of cases in the infected region with the long incubation period and meteorological factors along with latitude.
Results
The length of the mean short and long incubation periods for the cases were 25.5 days and 329.4 days, respectively. The total number of the study subjects was 897, and the number cases of short and long incubation periods was 575 (64.1%) and 322 (35.9%), respectively. The aspect of incubation period showed a significant difference by region of infection; there was a higher proportion of long incubation period infection cases in Gangwon-do than in Gyeonggi-do and Incheon. The proportion of long incubation period cases showed significant correlation with latitude and temperature of August and September of the infected regions.
Malaria is a mosquito-borne infectious disease of humans and other animals caused by a parasite of the genus Plasmodium [1, 2]. Malaria in humans is caused primarily by four species of Plasmodium: Plasmodium falciparum, Plasmodium vivax, Plasmodium ovale, and Plasmodium malariae; in addition, it can be caused by Plasmodium knowlesi, which was recently discovered in Malaysia [1, 2]. P. vivax malaria has lower fatality compared with P. falciparum malaria, but its occurrence is widespread across tropical, subtropical, and temperate regions [3].
In Korea, P. vivax malaria was an endemic disease before the 1970s, and its elimination was declared in 1979. It re-emerged, however, in 1993. In the initial stages of re-emergence, it occurred mostly in regions near the Demilitarized Zone (DMZ), such as Paju-si and Yeoncheon-gun, but it rapidly spread to the east and west. Recently, the incidence of malaria has increased notably in the Northwestern region of Gyeonggi-do, such as Gimpo-si, Paju-si, and Goyang-si, as well as in Incheon (Fig. 1) [3-5].
The incubation period of malaria appears to vary according to the type of parasites. In P. vivax malaria, strains from tropical regions show an onset of symptoms 2-3 weeks after infection, whereas strains from temperate regions show both a short incubation period (early primary attack, onset within 1 month after infection) and a long incubation period (late primary attack, onset 8-12 months after infection) [3]. This is attributable to the fact that, after invading liver cells, some strains of P. vivax malaria become a hypnozoites rather than schizonts; in such cases, relapse or long incubation periods (late primary attack) can occur after a certain period even when the parasites have completely disappeared from the blood [6]. It is presumed that, even with strains of the same P. vivax malaria, onset can differ according to varying proportions of sporozoites morphing into either hypnozoites or schizonts, which grow and multiply right away [7].
The spread of P. vivax malaria to other regions of Korea is mainly attributable to residents from P. vivax malaria non-risk regions visiting risk regions for military service, travel (visiting, business), or other purposes and being infected with latent hypnozoites, causing late primary attack [3]. As a result, cases occur even at times when vector mosquitoes are not active, causing difficulties in extermination, case management, and treatment of P. vivax malaria in Korea.
In Korea, the proportion of P. vivax malaria cases with long incubation periods is estimated at 50%. Therefore, it is necessary to analyze the onset of such cases for effective preventive measures and case management [8]. Previous research on the incubation period of P. vivax malaria has mostly focused on estimating the mean incubation period using a small number of cases over a short period [9-14], due to the difficulty of accurately identifying the time at which the incubation period begins following the bite of a malaria-infected mosquito.
This study used long-term epidemiological data regarding P. vivax malaria cases on a national scale to epidemiological investigate the influence on incubation period of various factors: demographic factors, clinical symptoms, time of infection and symptom onset, and environmental factors. This study aims to provide basic information that will help in establishing management plans for P. vivax malaria, which is one of the most important public health problems among vector-borne diseases.
The Korean Centers for Disease Control and Prevention (KCDC) established the National Notifiable Disease Surveillance System (NNDSS) in 2000, and since 2001 it has been compulsory for medical clinics and hospitals to notify the public health center when a national notifiable infectious disease. The public health center then reports this to KCDC. Among these diseases, malaria is a group-3 national notifiable infectious disease. Only confirmed cases are subject to the reporting requirement.
For malaria cases reported by medical clinics and hospitals, an infectious disease officer from the local public health center conducts an epidemiological investigation regarding personal information, clinical symptoms, risk factors for malaria infection within the past 2 years, and other cases characteristics through a telephone or face-to-face interview; this is based on the "Law on the Prevention and Control of Infectious Disease." The content of the epidemiological investigation conducted by the public health center is reviewed by the metropolitan infectious disease officer (province) and then reported to the Division of Epidemic Intelligence Service within KCDC. The officer in the Division of Epidemic Intelligence Service reviews the epidemiological investigation reported from the city/county to fix any errors and finally determines the infection pathway and region of the case.
To estimate the route and region of P. vivax malaria infection in Korea, 22 city/county/districts are designated and managed as P. vivax malaria risk regions. Considering the long incubation period of P. vivax malaria, regions with concentrated occurrence of P. vivax malaria during the last 2 years were Gangwon-do (Goseong-gun, Yanggu-gun, Inje-gun, Cheorwon-gun, Chuncheon-gun, Hwacheon-gun), Gyeonggi-do (Gapyeong-gun, Goyang-si, Gimpo-si, Dongducheon-si, Yangju-si, Yeoncheon-gun, Uijeongbu-si, Paju-si, Pocheon-si), and Incheon (Ganghwa-gun, Seo-gu, Jung-gu, Dong-gu, Ongjin-gun). Risk regions were determined by identifying risk factors such as residence, work, military service, and travel in the region. Other past infections or relapses/re-infections, as well as cases where the infection pathway was unidentified, were defined as unknown.
This study used data from a malaria epidemiological investigation conducted from 2001 to 2010, selecting for analysis those cases for which the time of infection could be estimated among civilian cases infected with P. vivax malaria in Korea.
Following precedent studies [9, 10], the mean incubation period was estimated by selecting only cases for which the start and end dates of the travel were accurately recorded and for which the travel duration to P. vivax malaria risk regions was within 30 days. Cases for which only the month of the travel was recorded or for which the travel duration was longer than 30 days were excluded from estimating the mean incubation period; even though classification of incubation period is possible in such cases, accurate confirmation of infection time is difficult. To obtain a higher degree of precision, the mean short incubation period estimation, only cases for which the travel duration was within 2 days were used, and for the mean long incubation period, extreme values (99 weeks or more after the infection) were excluded.
The mid-date of the stay was assumed to be the time of exposure and the incubation period was obtained based on the difference between date of onset. Based on the estimated mean incubation period, cases were classified as short incubation period or long incubation period.
Among civilian cases in Korea, a comparison and analysis were conducted on cases for whom the infection time could be estimated in order to examine the effect on incubation period of demographic factors, clinical symptoms and environmental factors such as meteorological factors or latitude of P. vivax malaria infection regions (region that is infected with malaria in an at-risk region).
Categorical variables (sex, infected region, clinical symptoms) were compared using the Chi-square test. Continuous variables (age) were compared using the Student's t-test.
To identify the correlation between meteorological factors in the region of infection and long incubation period cases, the proportion of such cases in each P. vivax malaria risk region was estimated for the 10 years from 2001 to 2010 (city/county/district), and a Pearson's correlation analysis was performed using the monthly mean meteorological factors (temperature, precipitation) of the relevant regions. For meteorological factors, processed data based on actual data measured by the Korea Meteorological Administration were used.
To identify the correlation between the latitude of the region of infection and incubation period, a Pearson's correlation analysis was performed with the proportion of long incubation cases over 10 years and the latitude of the region of infection. Latitudes were applied by designating the city or county office as the reference point.
Statistical analyses were performed with SPSS for Windows (version 12.0K; SPSS Inc., Chicago, IL). All tests of significance were two-tailed, and a P-value of less than 0.05 was considered statistically significant. Non-responses were excluded from the analysis.
Among the 12,253 cases with complete epidemiological data regarding malaria from 2001 to 2010, 1,181 cases were cases who had lived 2 years or more in P. vivax malaria non-risk regions and were presumed to be infected by P. vivax malaria through traveling to risk regions in Korea. Among these, those for whom the starting and ending dates were not recorded or unclear (N = 166), as well as those who had visited risk regions more than once (N = 50), were excluded. However, if various P. vivax malaria risk regions were visited within 1 month, it was regarded as one travel, and such cases were included; even though the region of infection could not be estimated, the incubation period could be differentiated. Cases who had a history of foreign travel between infection and symptom onset (N = 68) were excluded, as foreign travel could directly or indirectly influence the onset of P. vivax malaria. Hence, a total of 897 cases were selected as final including subjects (Fig. 2).
Among the 307 cases with a travel duration of within 30 days, the accuracy of estimating the mean short incubation period was heightened by using 184 cases with travel durations of within 2 days. The mean long incubation period was estimated using 45 cases, excluding 4 extreme value cases. The mean short incubation period length was 25.5 days (SD = 27.9 days). The mean long incubation period length was 329.4 days (SD = 37.7 days). Most cases showed symptom onset within 1 month of infection and then sharply declined, showing no onset for 4 to 7 months, increasing again after 8 to 13 months (Fig. 3). Based on this, the period between 25 and 33 weeks, when there was no case, was used to establish cut-off points; symptom onset before 25 weeks post-infection was defined as a short incubation period, and symptom onset after 33 weeks post-infection was defined as a long incubation period.
Among the 897 cases whose incubation periods could be confirmed from 2001 to 2010, there were 575 (64.1%) short incubation period cases and 322 (35.9%) long incubation period cases. Thus, the proportion of short incubation cases appeared to be higher (Table 1).
Incubation period showed differences according to sex and the region of infection. The proportion of females was significantly higher among long incubation cases compared with short incubation cases (P = 0.041). Infections occurring in Gangwon-do had a significantly higher proportion of long incubation cases compared to Incheon and Gyeonggi-do (P = 0.001) (Table 1).
Over time, the proportion of infections occurring in Gangwon-do decreased while those occurring in Incheon increased, and an increasing trend in the proportion of short incubation cases was observed (Fig. 4).
There were no significant differences observed in clinical symptoms, such as fever or chills, between the long and short incubation cases (Table 2). The length of diagnosis, from the date of symptom onset to the confirmed date, was on mean 10.5 days (SD = 7.9 days) for long incubation period cases, about 1.7 days longer than the 8.8 days (SD = 6.7 days) for short incubation period cases (P = 0.0012).
Incubation period showed significant differences according to the time of infection and symptom onset (P < 0.001). The infection time peaked from June to August, when the vector mosquito is active, and sharply declined after August (Fig. 5A). Among the 227 cases from March to June, 195 (85.9%) had short incubation periods; thus, it was confirmed that there were more cases with short incubation periods when the infection period was earlier. Among the 466 cases from June to August, 288 (61.8%) appeared to have short incubation periods.
Regarding onset time, there were more long incubation period cases, with infection having occurred the previous year, among cases with symptom onset from April to June. However, there was a sharp decline in long incubation periods and an increase in short incubation periods for cases with symptom onset after July (Fig. 5B).
Table 3 shows the correlation between the proportion of long-incubation-period cases for each P. vivax malaria infected region and mean monthly meteorological factors from 2001 to 2010. The proportion of long incubation period cases showed a positive correlation with precipitation in October (r = 0.51, P = 0.045), a negative correlation with mean temperature in September and August, and a negative correlation with minimum temperature in September (respectively, r = -0.52, P = 0.040; r = -0.51, P = 0.045; r = -0.500, P = 0.049) (Table 3).
Figure 5 shows the correlation between latitude and the proportion of long incubation period cases for each P. vivax malaria infected region, and a high positive correlation was observed (r = 0.73, P = 0.001) (Fig. 6). The proportion of long incubation cases appeared to be high in high-latitude regions, such as Goseong and Inje-gun in Gangwon-do and Yeoncheon-gun in Gyeonggi-do.
This study used epidemiological data regarding P. vivax malaria cases in Korea from 2001 to 2010 to classify cases infected while visiting risk regions into long and short incubation periods. Then, we analyzed the correlation between incubation period and demographic characteristics, time of infection and symptom onset, and environmental factors such as latitude and meteorological factors. The results showed that the mean incubation period for short incubation period cases, with an onset of within 1 month, was 25.5 days. The mean incubation period for long incubation period cases was 329.4 days. These results are similar to those of precedent studies [9, 10].
The 10-year mean proportion of long incubation periods was approximately 36%, which is lower than the long incubation period proportion of 75% reported in a P. vivax malaria study that investigated a North Korean strain in Moscow from 1953 to 1972 [9]. However, it is similar to the results of recent studies of P. vivax malaria cases in Korea [10, 11].
In this study, incubation period appeared to be associated with the region of infection, with infections presumed to have occurred in Gangwon-do having a higher proportion of long incubation periods compared with infections presumed to have occurred in Gyeonggi-do or Incheon. According to precedent studies, since 2005, cases have been concentrated in the western P. vivax malaria risk regions of Ganghwa-gun, Seo-gu, Jung-gu and Dong-gu in Incheon and Goyang-si, Gimpo-si, and Paju-si in Gyeonggi-do; meanwhile, there have been very few cases in Gangwon-do other than in Cheorwon-gun [3]. Our study also showed that the number of cases infected in Gangwon-do gradually decreased while those infected in Incheon increased, and the proportion of short incubation periods also increased. It is expected that this trend will continue in the future and, presumably, have an effect on the increase of short incubation period cases.
Differences in incubation period were observed according to the time of infection and symptom onset. A precedent study that investigated aspect of symptom onset among soldiers who entered after November that short incubation cases occurred from early June [4]. In our study, however, although there were a small number of cases, 123 cases were presumed to be infected from March to May, and 103 cases (83.7%) showed a short incubation period. Infections during this period showed symptom onset from May to August, or 2 to 4 months later, rather than within 1 month of infection.
In precedent studies, cases with symptom onset before May were all classified as having long incubation periods [8], but our results included 26 cases with symptom onset before May who were presumed have short incubation periods. The KCDC's vector mosquito (Anopheles sinensis) survey is conducted at 27 stations in the P. vivax malaria risk regions (Gangwon-do 6, Gyeonggi-do 12, Incheon 9) from April to October every year. In the vector mosquito (Anopheles sinensis) survey results from 2007-2011, the mean first collection time was late May, while the appearance of vector mosquitos infected with parasites started from mid-July [15]. However, the vector mosquitoes of some regions had been collected as early as the first week of April [16], and in a military investigation, mosquitoes infected with the P. vivax malaria parasite were reported to appear in May, 2 months earlier than in the investigation of civilians [15].
The risk of being infected by P. vivax malaria is low from March to May, but there were a considerable number of cases who responded that they had visited the P. vivax malaria risk regions in those months, without any other risk factors for exposure to the vector mosquito of P. vivax malaria outside this time period. If they were not infected by P. vivax malaria during this period, it is possible that the P. vivax malaria non-risk regions in which they reside have, in fact, become risk regions. However, in the 'Surveillance of vector mosquitoes and investigation of P. vivax malaria parasites,' conducted in non-risk regions near the P. vivax malaria risk regions by the KCDC in 2012, P. vivax malaria vector mosquitoes were found to exist in non-risk regions, but no mosquitoes infected with the P. vivax malaria parasite were discovered [17]. Further research is needed, but it is currently assumed that there is a low risk of infection in regions near the P. vivax malaria risk regions.
As a limiting factor, subjects in our research who travel to P. vivax malaria risk regions as the cause of their infections, which were not linked to mosquito survey data. Hence, there is a need for additional research studies, such as vector mosquito monitoring and epidemiologic investigations, to examine whether these results are due to errors in memory or whether, as precedent studies have suggested, vector mosquitoes have begun to appear earlier and with longer periods of activity due to climate change, creating conditions favorable for P. vivax malaria transmission [18, 19].
In addition, among cases infected in June, only about 20% were long incubation period cases, whereas about 40% of infections occurring in July or August had long incubation periods despite the fact that the temperature and precipitation conditions were sufficient for the onset of P. vivax malaria. Hence, there is a need for further research.
This study analyzed the correlation between the mean proportion of long incubation period cases and monthly meteorological factors for each infected region from 2001 to 2010, with the following results. The proportion of long incubation-period cases showed a tendency to increase with increasing precipitation in October, with lowering mean temperature in September, and with lowering minimum temperatures in August and September. From 2005-2006, the highest 10-days occurrence of P. vivax malaria extended to early September; this increase of patients in September and October is considered to result from an increase in early-primary-attack cases due to short incubation period cases [5]. If the temperature in September increases in the future for reasons such as climate change, the malaria transmission period will be extended; in this case, it is expected that infections occurring in late August to early September will show short rather than long incubation periods, with the onset of symptoms developing by late October.
Among the strains of Plasmodium, only P. vivax malaria has a short incubation period in low-latitude tropical regions and both long and short incubation periods in subtropical regions. It is known that the proportion of short and long incubation periods depends on latitude [20]. In addition, P. vivax hibernans, the strain found in high-latitude regions such as Russia, is known to have only a long incubation period [20]. White [21] predicted that as the latitude increases, malaria cases with short incubation periods decline given that the mosquito breeding seasons shorten. Our results showed a clear positive correlation between the 10-year mean proportion of long incubation periods and the latitude of the relevant P. vivax malaria risk region in Korea. The difference in latitude between such P. vivax malaria risk regions in Korea is not big, but high-latitude regions such as Goseong-gun, Inje-gun, and Cheorwon-gun in Gangwon-do and Yeoncheon-gun in Gyeonggi-do are considered to have considerably different meteorological conditions, such as temperature in winter, compared with the low-latitude regions of Goyang-si in Gyeonggi-do and Incheon. Using the annual climatological report issued by the Korea Meteorological Administration, the mean and minimum temperatures of P. vivax malaria risk regions with weather stations were compared. Comparing Cheorwon-gun in Gangwon-do and Incheon revealed that the mean annual temperature over 10 years (2001-2010) was 2.5℃ lower in Cheorwon-gun compared with Incheon, while the mean annual minimum temperature was about 4.6℃ lower in Cheorwon-gun compared with Incheon. The difference in mean monthly temperature was clearer, and the difference in both mean and minimum temperature was biggest from January to March. In particular, the 10-year mean minimum temperature in January showed a difference of 7.5℃ [22]. Various explanations have been proposed for the two different incubation periods of P. vivax malaria [23], but the cause is likely not a single factor but rather a complex interaction of environmental factors, such as the meteorological conditions, latitude of risk regions, the principal vector's biological characteristics, adaptation to the environment, and characteristics of the infected cases.
The limitations of this study are as follows. First, the most important element in any incubation period study is having an accurate estimation of the time of infection, but this epidemiological investigation had to rely on the memory of individuals; thus, there could be an error in the travel histories. Second, as this research only investigated P. vivax malaria cases who had been infected during short-term visits, there were a small number of cases; this factor may have limited our ability to explain the incubation period of all P. vivax malaria cases in the country. However, as the purpose of the research was to identify the factors affecting incubation period, it was not necessary to examine all cases. The last limitation is that the cause-and-effect relationship cannot be confirmed using only cross-tabulation and correlation analysis, leaving open the possibility that the results happened by chance.
This study is significant in that it is the first study to comprehensively consider environmental and epidemiological factors influencing the incubation period of P. vivax malaria cases in Korea. In terms of factors that affected long and short incubation periods, results showed that there were significant differences according to the region of infection, time of infection, and time of symptom onset. In addition, there was a high positive correlation with the latitude of the region of infection, and there was a correlation with temperature in August and September. It is anticipated that P. vivax malaria cases with short incubation periods will increase in the future. Increasing proportion of patients with short incubation periods may be convenient for case management and treatment perspective, but there will be a need to improve vector mosquito control as the transmission period of P. vivax malaria extends. In addition, there is a need for additional research to comprehensively examine the vector mosquito (Anopheles sinensis), parasite strains, and epidemiologic investigation period in order to identify differences in the incubation period of P. vivax malaria in Korea.
Figures and Tables
Figure 1
Major risk areas of P. vivax malaria in Korea (2010-2011).
In 1990s, initial stages of re-emergence, it occurred mostly in regions near the Demiliatarized Zone (DMZ), but it rapidly spread to the east and west. Recently, the incidence of malaria has increased in Northwestern region of Gyeonggi-do, such as Gimpo-si, Paju-si, Goyang-si, and Incheon.
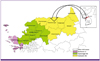
Figure 3
Distribution of the short and long incubation periods of P. vivax malaria cases, 2001-2010.

Figure 4
Yearly proportin of malaria infected regions and patients with short incubation period, 2001-2010.
Gagnwon-do: Goseong-gun,Yanggu-gun,Inje-gun, Cheorwon-gun, Chuncheong-gun, Hwacheon-gun.
Gyeonggi-do: Gapyeong-gun, Goyang-si, Gimpo-si, Dongducheon-si, Yangju-si, Yeoncheon-gun, Uijeongbu-si, Paju-si, Pocheonsi.
Incheon: Ganghwa-gun, Seo-gu, Jung-gu, Dong-gu, Ongjin-gun.

Figure 5
(A) Monthly distribution of cases with P. vivax malaria by infection time, 2001-2010. (B) Monthly distribution of cases with P. vivax malaria by disease onset time, 2001-2010.

Figure 6
Correlation between proportion of P. vivax malaria with long incubation period and latitude in infected regionsa, 2001-2010.
aGoseong-gun, Inje-gun, Cheolwon-gun, Chuncheon-gun, Hwacheon-gun, Gapyeong-gun, Goyang-si, Gimpo-si, Dongducheon-si, Yeoncheon-gun, Paju-si, Pocheon-si, Ganghwa-gun, Seo-gu, Jung-gu, Ongjin-gun.

Table 1
Demographic characteristics of P. vivax malaria cases, 2001-2010

The data were expressed as number (%).
aAge data was normally distributed and analyzed with Student's t-test.
bCategorical data (sex, infected region) were calculated by Chi-square test.
cGagnwon-do: Goseong-gun,Yanggu-gun,Inje-gun, Cheorwon-gun, Chuncheong-gun, Hwacheon-gun.
dGyeonggi-do: Gapyeong-gun, Goyang-si, Gimpo-si, Dongducheon-si, Yangju-si, Yeoncheon-gun, Uijeongbu-si, Paju-si, Pocheon-si.
eIncheon: Ganghwa-gun, Seo-gu, Jung-gu, Dong-gu, Ongjin-gun.
fCases with whose infected regions are not identified.
References
1. Sinden R, Gilles H. Warrell DA, Gilles HM, editors. The malaria parasites. Essential malariology. 2002. 4th ed. New York: Oxford University Press;8–34.
2. Cox-Singh J, Singh B. Knowlesi malaria: newly emergent and of public health importance? Trends Parasitol. 2008. 24:406–410.

3. Yeom JS, Park JW. Status of vivax malaria after re-emergence in South Korea. Infect Chemother. 2008. 40:191–198.

4. Park JW, Klein TA, Lee HC, Pacha LA, Ryu SH, Yeom JS, Moon SH, Kim TS, Chai JY, Oh MD, Choe KW. Vivax malaria: a continuing health threat to the Republic of Korea. Am J Trop Med Hyg. 2003. 69:159–167.

5. Park JW. Status of Plasmodium vivax malaria in the republic of Korea after reemergence. Hanyang Med Rev. 2010. 30:176–186.

7. Shute PG, Lupascu G, Branzei P, Maryon M, Constantinescu P, Bruce-Chwatt LJ, Draper CC, Killick-Kendrick R, Garnham PC. A strain of Plasmodium vivax characterized by prolonged incubation: the effect of numbers of sporozoites on the length of the prepatent period. Trans R Soc Trop Med Hyg. 1976. 70:474–481.

8. Korea Centers for Disease Control and Prevention. Interim evaluation of efficacy of 2010 malaria eradication project. 2008. Seoul, Korea: KCDC;9–72.
9. Tiburskaya NA, Vrublevskaja OS. The course of infections caused by the North Korean strain of Plasmodium Vivax. 1977. Geneva: WHO;1–19.
10. Nishiura H, Lee HW, Cho SH, Lee WG, In TS, Moon SU, Chung GT, Kim TS. Estimates of short- and long-term incubation periods of Plasmodium vivax malaria in the Republic of Korea. Trans R Soc Trop Med Hyg. 2007. 101:338–343.

11. Nah K, Choi I, Kim Y. Estimation of the incubation period of P.vivax Malaria in Korea from 2006 to 2008. J Korean Data Inf Sci Soc. 2010. 21:1237–1242.
12. Oh MD, Shin H, Shin D, Kim U, Lee S, Kim N, Choi MH, Chai JY, Choe K. Clinical features of vivax malaria. Am J Trop Med Hyg. 2001. 65:143–146.

13. Warwick R, Swimer GJ, Britt RP. Prolonged incubation period of imported P. vivax malaria in London. J R Soc Med. 1980. 73:333–336.

14. Shute PG. Latency and long-term relapses in benign tertian malaria. Trans R Soc Trop Med Hyg. 1946. 40:189–200.

15. Yoo DH, Park MY, Shin E-H, Chang KS. Malaria vector mosquitoes in nine Koran army-base camps near Dimilitarized zone in the Republic of Korea. Am J Trop Med Hyg. 2013. [In press].
16. Korea Centers for Disease Control and Prevention. The white book on infections disease control. 2012. Osong, Korea: KCDC;381–382.
17. Korea Centers for Disease Control and Prevention. Surveillance of vector mosquitoes and investigation of vivax parasites in non-endemic areas in Korea. 2012. Osong, Korea: KCDC;2–40.
18. Park JW. Changing transmission pattern of Plasmodium vivax malaria in the Republic of Korea: relationship with climate change. Environ Health Toxicol. 2011. 26:e2011001.
19. Martens P, Kovats RS, Nijhof S, de Vries P, Livermore MT, Bradley DJ, Cox J, McMichael AJ. Climate change and future populations at risk of malaria. Global Environmental Change. 1999. 9:S89–S107.

20. Bray RS, Garnham PC. The life-cycle of primate malaria parasites. Br Med Bull. 1982. 38:117–122.

21. White NJ. Determinants of relapse periodicity in Plasmodium vivax malaria. Malar J. 2011. 10:297.
22. Korea Meteorological Administration. Annual Climatological Report 2001~2010. 2010. Seoul, Korea: KMA.
23. Brasil P, de Pina Costa A, Pedro RS, da Silveira Bressan C, da Silva S, Tauil PL, Daniel-Ribeiro CT. Unexpectedlylongincubationperiod of Plasmodium vivax malaria, in the absence of chemoprophylaxis, in patients diagnosed outside the transmission area in Brazil. Malar J. 2011. 10:122.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download