Abstract
Syphilitic myelitis is a rare manifestation of neurosyphilis, whose magnetic resonance imaging findings are not well documented. The authors report on a case of a 48-year-old male who presented with acute onset of paraplegia and voiding difficulty and was diagnosed as having syphilitic myelitis. Among tests performed for the diagnosis, serum Venereal Disease Research Laboratory (VDRL) and fluorescent treponemal antibody absorbed (FTA-ABS) tests showed a positive result. Analysis of cerebrospinal fluid (CSF) showed a normal white blood cell count, increased protein, reactive VDRL, and FTA-ABS tests. Magnetic resonance imaging (MRI) of cervical and thoracic spines showed diffuse intramedullary T2-hyperintense signal intensity without T1-weighted gadolinium enhancement. The syphilitic myelitis was resolved after institution of intravenous high dose penicillin G therapy for two weeks. Additional follow-up CSF analysis performed three months after treatment showed decreased protein and negative VDRL. MRI taken nine months later appeared normal and VDRL in CSF was still negative. This case study reports on the first Korean case of acute transverse myelitis caused by syphilis.
Neurosyphilis refers to an infection of the central nervous system (CNS) caused by Treponemapallidum. The disease can be asymptomatic or manifest as various disorders, such as tabes dorsalis, general paresis, and meningovascular syphilis. Syphilitic myelitis is extremely rare, and might be misdiagnosed as acute transverse myelitis, a form of meningo-vascular syphilis with abnormalities confined to the spinal cord [1].
Only a few reports have been published. Here we report on a case of syphilitic myelitis associated with neurosyphilis.
A 48-year-old male was admitted because of bilateral leg weakness with numbness and voiding difficulty, which started four days before admission. The patient was almost unable to walk unsupported. He had no underlying disease. He had been divorced for 10 years, and has not had sexual intercourse after the last occasion with his girlfriend three years ago. He denied any history of hospital admission, drug allergy, or recent vaccination.
Vital signs at admission were as follows: blood pressure, 130/70 mmHg; pulse rate, 70 beats/min; respiratory rate, 20 breaths/min; and body temperature, 36.7℃. He appeared to be chronically ill. He was mentally alert and did not have any cognitive or memory disturbance. Both pupils showed normal light reflex. A neurological examination showed reduced muscle strength in both legs but normal arm motor function. Motor function grades were 4/5 in the right leg and 3/5 in the left leg. Knee jerk and ankle jerk reflexes were increased, and pinprick and thermal senses were absent below the T10 level. Joint position and vibration senses in the legs were intact. No pathological Babinski sign was observed in the feet. Straight-leg raising to an angle of 80 degrees for the right leg and 90 degrees for the left leg were noted. The patient did not have skin ulceration, mucous patches, or gummas on genitalia.
Complete blood cell counts and chemical tests, including liver enzymes, were within normal ranges. Urinalysis showed no abnormal findings. The Venereal Disease Research Laboratory (VDRL) and fluorescent treponemal antibody-absorbed (FTA-ABS) tests in serum were positive. He was negative for hepatitis B surface antigen (HBs Ag) and Human immunodeficiency virus (HIV) antibody. Chest radiography and an electrocardiogram showed no abnormal findings.
A lumbar puncture showed an opening cerebrospinal fluid (CSF) pressure of 18 cmH2O. Results of CSF analysis showed a white blood cell (WBC) count of 0 cells/mm3, a red blood cell (RBC) count of 17,500 cells/mm3, a protein level of 289 mg/dL, and a glucose level of 111 mg/dL (the synchronous serum value was 158 mg/dL). The CSF, VDRL, and FTA-ABS IgG tests were reactive (Table 1).
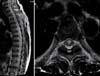
Magnetic resonance imaging (MRI) of the cervical and thoracic spines showed intramedullary T2-hyperintense signal intensity in the ventral spinal cord from the lower cervical spines to the lumbar spines, without T1-weighted enhancement (Fig. 1).
A neuro-ophthalmic examination showed no edema in the retina and Argyll Robertson (AR) pupils. Findings on an echocardiogram were normal.
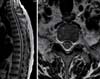
Under the diagnosis of syphilis myelitis, high dose penicillin G was administered intravenously at a daily dose of 4 million IU every four hours for two weeks. Prednisolone (30 mg) was also given for avoidance of the Jarisch-Herxheimer reaction. After initiation of treatment, the patient's leg strength returned to normal. At three months and nine months post diagnosis, CSF analysis showed normal protein and a non-reactive VDRL test (Table 1). Findings on follow up MRI of the thoracic spine taken after nine months were normal (Fig. 2).
Syphilis is a sexually transmitted infectious disease caused by the spirochete T.pallidum [2]. Although it can be involved in any stage of the disease, the CNS is primarily affected in the late state. The incidence of neurosyphilis is estimated to be 5-10% in untreated cases and up to 30% in patients who progress to the late stage [3].
Neurosyphilis can be divided into four clinical types: meningeal, vascular, general paresis, and tabes dorsalis, based on clinical symptoms and the time elapsed between primary infection and the appearance of symptoms. Although tabes dorsalis is the most well-known spinal form of neurosyphilis, other spinal abnormalities, including meningomyelitis, pachymeningitis, spastic paraparesis, and amyotrophy, have been reported [1]. Syphilitic myelitis is a rarer manifestation of neurosyphilis than meningomyelitis. Diagnosis of syphilitic myelitis is based on symptoms of myelopathy, a positive serologic test for neurosyphilis, and spine MRI. In the MRI, abnormalities are confined to the spinal cord, which is a differential point from other forms of neurosyphilis [1]. Diagnosis of syphilitic myelitis is difficult, as it mimics idiopathic transverse myelitis, spinal cord infarction, and acute disseminated encephalomyelitis (ADEM) [4]. Therefore, all patients with unclear myelopathy should undergo serologic testing for syphilis.
MRI findings in syphilitic myelitis are infrequently reported. Tashiro et al. first described MRI findings in syphilitic myelitis in 1987, and four cases have since been documented in the literature [5-9]. Short segment high signal intensity in the thoracic cord on T2-weighted images and abnormal enhancement, predominantly in parts of the superficial spinal cord, may be observed on gadolinium-enhanced images [5, 7, 8]. These abnormalities of the spinal cord are probably the result of meningeal inflammation and spinal cord ischemia [5, 8, 10, 11]. Spinal cord lesions have been reported to resolve completely following treatment in these patients, and the disappearance of high-signal lesions may reflect the reversibility of ischemic or inflammatory changes [5, 8].
Tsui et al. reported different MRI findings for syphilitic myelitis of extensive central high signal intensity involving the whole cord on T2-weighted MRI images, and focal nodular enhancement in the dorsal aspect of the middle thoracic cord at the T8-T9 level [9]. Chilver-Stainer et al. reported high signal intensity of the central portion of the spinal cord parenchyma below T6 on T2-weighted images and two focal enhancements on T1-weighted images [6].
The major lesion in syphilitic myelitis is on the thoracic spine [5, 7, 8], although Tsui et al. reported on a patient whose MRI showed diffuse high signal intensity in the whole spinal cord on T2-weighted images [9].
In our case, unusual MRI findings were observed in T2-weighted MR images, which showed diffuse high signal intensity from the lower C spine level to the L spine level without enhancement on T1-weighted images. The high-signal lesions were diffuse and presented along the whole spinal cord without enhancement, in contrast to previous reports of short or long segmental hyperintensity with cord enhancement.
Our patient was treated with intravenous aqueous penicillin G (24 million IU per day) for two weeks. Over the years, high doses of intravenous (IV) aqueous (18 to 24 million units per day, administered as 3 to 4 million units IV every four hours, or 24 million units daily as a continuous infusion) for 10 to 14 days have been recommended as first-line therapy for treatment of neurosyphilis [1]. However, no controlled trials have evaluated the efficacy of the currently used forms of penicillin for treatment of neurosyphilis.
We report on the first Korean case of syphilitic myelitis with unusual MRI findings in a HIV-negative patient. Because the clinical and imaging findings of this disease are non-specific, this potentially treatable disease entity should be included in the differential diagnosis of acute transverse myelitis. A high index of suspicion is required in order to make the diagnosis. In addition, these diagnoses should be kept in mind in patients presenting with an acute neurologic deficit. Serum and CSF VDRL and FTA-ABS tests are mandatory in these patients.
Figures and Tables
Figure 1
T2 weighted image obtained by T-spine MRI showing intramedullary high signal intensity from the lower C-spine to L1, suggestive of non-tumorous myelopathy. (A) Sagittal image showing long segmental signal change of the spinal cord. (B) Axial image showing the concentric locations of lesions.
References
2. Dattner B, Thomas EW, De Mello L. Criteria for the management of neurosyphilis. Am J Med. 1951. 10:463–467.

3. Harris DE, Enterline DS, Tien RD. Neurosyphilis in patients with AIDS. Neuroimaging Clin N Am. 1997. 7:215–221.
4. Kikuchi S, Shinpo K, Niino M, Tashiro K. Subacute syphilitic meningomyelitis with characteristic spinal MRI findings. J Neurol. 2003. 250:106–107.

5. Tashiro K, Moriwaka F, Sudo K, Akino M, Abe H. Syphilitic myelitis with its magnetic resonance imaging (MRI) verification and successful treatment. Jpn J Psychiatry Neurol. 1987. 41:269–271.

6. Chilver-Stainer L, Fischer U, Hauf M, Fux CA, Sturzenegger M. Syphilitic myelitis: rare, nonspecific, but treatable. Neurology. 2009. 72:673–675.

7. Matijosaitis V, Vaitkus A, Pauza V, Valiukeviciene S, Gleizniene R. Neurosyphilis manifesting as spinal transverse myelitis. Medicina (Kaunas). 2006. 42:401–405.
8. Nabatame H, Nakamura K, Matuda M, Fujimoto N, Dodo Y, Imura T. MRI of syphilitic myelitis. Neuroradiology. 1992. 34:105–106.

9. Tsui EY, Ng SH, Chow L, Lai KF, Fong D, Chan JH. Syphilitic myelitis with diffuse spinal cord abnormality on MR imaging. Eur Radiol. 2002. 12:2973–2976.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download