Abstract
On the 12th day of abacavir treatment, a 39-year old HIV-infected male patient was admitted with fever, generalized rash, abdominal pain, and watery diarrhea that had persisted for five days. Results of blood tests indicated rapid progression of hepatitis and renal failure. The day after stopping anti-retroviral therapy, his fever subsided and his liver function began to normalize. He was clinically diagnosed with abacavir hypersensitivity and was found to carry the HLA-B*57:01 allele. This is the first reported case of abacavir hypersensitivity associated with the presence of the HLA-B*57:01 allele in Korea.
Abacavir is a nucleoside reverse-transcriptase inhibitor with activity against the human immunodeficiency virus (HIV). A hypersensitivity reaction, which occurs in approximately 5% of patients, is the major treatment-limiting toxicity of abacavir [1]. Abacavir hypersensitivity reaction is characterized by multisystem involvement, and, in rare cases, it can be fatal [2]. Median time to onset of symptoms of hypersensitivity is 11 days. Symptoms usually appear within at least the first six weeks [1, 2]. Common manifestations include fever, rash, constitutional symptoms, and gastrointestinal tract symptoms. Ninety six percent of patients have fever, rash, or both [1]. Less common manifestations include respiratory or musculoskeletal symptoms, headache, paresthesia, edema, renal failure, hepatic failure, and anaphylaxis. In particular, respiratory symptoms are more severe with continued dosing [1, 3]. Symptoms related to hypersensitivity show worsening with continued therapy and usually show improvement within 24 hours of discontinuation [1-3]. Subsequent use of abacavir typically results in a more severe, rapid, and potentially life threatening reaction; therefore, re-challenge is contraindicated [1-3].
Abacavir hypersensitivity is a major adverse effect and has been identified as a pharmacogenomic risk factor in association with usage of abacavir. However, no case of abacavir hypersensitivity associated with presence of the HLA-B*57:01 allele has been previously reported in Korea. We report on our experience with an HIV-infected patient carrying the HLA-B*57:01 allele who was diagnosed with abacavir hypersensitivity.
A 39-year-old male patient was admitted with fever, generalized rash, abdominal pain, and watery diarrhea for five days. He had recently been diagnosed with HIV infection. Because he had a low CD4+ cell count, 206 cells/mm3, anti-retroviral therapy with abacavir/lamivudine and lopinavir/ritonavir was initiated. He took abacavir/lamivudine once daily and lopinavir/ritonavir twice daily and did not take any other medications. Three hours after taking the first dose of abacavir/lamivudine, he experienced myalgia. After seven days, his body temperature rose to near 40℃ and he experienced abdominal pain and watery diarrhea. The symptoms showed progressive worsening and the time interval of administration of antipyretics shortened.
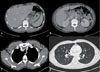
On admission, his blood pressure was 104/62 mmHg, heart rate was 126 beats/min, respiratory rate was 20 breaths/min, and body temperature was 39.1℃. No significant symptoms, other than a generalized skin rash and mild abdominal tenderness, were observed on physical examinations. White blood cell (WBC) count was 7,290/mm3, hemoglobin level was 15.1 g/dL, and platelet count was 85,000/mm3. Levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were elevated to 940 IU/L and 478 IU/L. Total bilirubin level was 1.8 mg/dL. Blood urea nitrogen level was 16.7 mg/dL and serum creatinine level was 1.45 mg/dL. HIV viral load was 180,000 copies/mL and CD4+ T-lymphocyte count was 305 cells/mm3. Antibodies to hepatitis C, hepatitis A, and hepatitis B surface antigens were absent. Findings on computed tomography (CT) scan of the chest indicated normal lung parenchyme and small axillar lymph nodes, and findings on a CT scan of the abdomen indicated hepatic congestion, gallbladder wall edema, and mild splenomegaly (Fig. 1). Results of blood and stool cultures were negative. Liver function showed rapid worsening; therefore, all medications were discontinued. The day after stopping antiretroviral agents, the patient's fever subsided and AST and ALT levels began to decrease (Table 1). Human leukocyte antigen (HLA)-B typing was performed. He was found to have the HLA-B*37:01 and B*57:01 alleles. He was believed to be suffering from abacavir hypersensitivity. Three weeks after stopping abacavir, he exhibited no symptoms and all laboratory findings were normalized. This is the first reported case of a patient with abacavir hypersensitivity associated with the HLA-B*57:01 allele in Korea.
This is the first reported case involving abacavir hypersensitivity in a Korean patient who was found to harbor the HLA-B*57:01 allele. On the seventh day after initiation of abacavir treatment, he developed a fever, abdominal pain, and watery diarrhea. Hepatitis and renal failure showed rapid progression. The day after cessation of abacavir intake, the patient's symptoms began to diminish. Within the next three months, he had made a full recovery from his hypersensitivity reaction and his laboratory findings were normal.
There are several clinical criteria for diagnosis of abacavir hypersensitivity. First, symptoms must occur within six weeks of the start of abacavir treatment. Second, more than two of the following symptoms must be observed: fever, rash, and gastrointestinal, constitutional, or respiratory symptoms. Third, symptoms should be alleviated within three days after cessation of abacavir treatment. Finally, the symptoms cannot be explained by other causes [1-3]. As observed in the present case, the patient fulfilled all of these criteria, including both the timing of the onset of symptoms and the actual symptoms observed.
Skin patch testing has been used successfully as an adjunct to clinical diagnosis to aid in identification of patients with immunologically-mediated abacavir hypersensitivity. One patch panel uses 1% and 10% concentrations of abacavir and excipient and petrolatum controls. It is applied to the mid-back and is removed 48 hours after placement. Results of the test are read within 24 hours after patch removal. A positive cutaneous reaction (erythema; induration; pruritus; and, in some cases, vesiculation and blistering) implies immunological confirmation of abacavir hypersensitivity [4, 5].
Both clinical diagnosis and skin patch testing have limitations. In clinical diagnosis, a rash may also be associated with use of efavirenz, and fever, rash, and hepatitis may also be associated with use of nevirapine, and gastrointestinal symptoms may be associated with use of protease inhibitors [3]. In the PREDICT-1 study, due to incidence of clinically diagnosed patients with the HLA-B*57:01 allele who demonstrated false-negative results on the epicutaneous patch test, a negative skin patch result could neither rule out abacavir hypersensitivity nor justify re-challenge [3].
In 2002, an association between diagnosis of hypersensitivity and presence of the major histocompatibility complex class I allele HLA-B*57:01 was reported independently by two research groups [2, 6]. In the PREDICT-1 study, HLA-B*57:01 screening was proven to reduce the risk of hypersensitivity reactions to abacavir [3]. Currently, all patients are recommended to undergo screening for the HLA-B*57:01 allele before receiving a prescription for abacavir.
Carriage frequencies of the HLA-B*57:01 allele in the United States are 5% among African-Americans and 8% among Caucasians: the prevalence of abacavir hypersensitivity is 4% among HIV-positive African-Americans and 5% among Caucasians [7, 8]. Previous studies have reported on a low prevalence of the HLA-B*57:01 allele in East-Asians; only one (0.3%) of 320 HIV-infected Taiwanese patients carries the allele [9]. Frequency of the HLA-B*57:01 gene in healthy Koreans is reported to be 0.2-0.3% [10] and has not been reported to occur among HIV-infected Koreans thus far. In 2009, Park et al, who investigated HLA-B*57:01 and abacavir hypersensitivity in Korean HIV-infected patients [11], found neither HLA-B*57:01 carrier nor immunologically confirmed abacavir hypersensitivity. They suggested that because of a low prevalence of the allele, HLA-B*57:01 screening in Asians might not be as useful. Therefore, despite the guidelines, most Korean patients have not undergone screening for the presence of the HLA-B*:57:01 allele prior to undergoing treatment with abacavir [12].
We agree that, due to the low frequency of HLA-B*57:01 and the cost of HLA typing, screening of all Korean patients before abacavir treatment is not necessary. However, all clinicians prescribing abacavir should have knowledge of the severe consequences of abacavir hypersensitivity and should also be aware that some patients carry the allele associated with abacavir hypersensitivity, even if at a very low frequency. Instead of screening, clinicians should be aware of the clinical manifestations of abacavir hypersensitivity, and, once it is suspected, HLA-B typing should be performed immediately.
The present case emphasizes the necessity of vigilance in monitoring Korean patients undergoing treatment with abacavir, as some patients may carry the allele, which is associated with abacavir hypersensitivity, even if at a very low prevalence.
Figures and Tables
References
1. Hetherington S, McGuirk S, Powell G, Cutrell A, Naderer O, Spreen B, Lafon S, Pearce G, Steel H. Hypersenstivity reactions during therapy with the nucleoside reverse transcriptase inhibitor abacavir. Clin Ther. 2001. 23:1603–1614.

2. Mallal S, Nolan D, Witt C, Masel G, Martin AM, Moore C, Sayer D, Castley A, Mamotte C, Maxwell D, James I, Christiansen FT. Association between presence of HLA-B*5701, HLA-DR-7, and HLA-DQ3 and hypersensitivity to HIV-1 reverse-transcriptase inhibitor abacavir. Lancet. 2002. 359:727–732.

3. Mallal S, Phillips E, Carosi G, Molina JM, Workman C, Tomazic J, Jägel-Guedes E, Rugina S, Kozyrev O, Cid JF, Hay P, Nolan D, Hughes S, Hughes A, Ryan S, Fitch N, Thorborn D, Benbow A. PREDICT-1 Study Team. HLA-B*5701 screening for hypersensitivity to abacavir. N Engl J Med. 2008. 358:568–579.

4. Saag M, Balu R, Phillips E, Brachman P, Martorell C, Burman W, Stancil B, Mosteller M, Brothers C, Wannamaker P, Hughes A, Sutherland-Phillips D, Mallal S, Shaefer M. Study of Hypersensitivity to Abacavir and Pharmacogenetic Evaluation Study Team. High sensitivity of human leukocyte antigen-B*5701 as a marker for immunologically confirmed abacavir hypersensitivity in white and black patients. Clin Infect Dis. 2008. 46:1111–1118.

5. Phillips EJ, Sullivan JR, Knowles SR, Shear NH. Utility of patch testing in patients with hypersensitivity syndromes associated with abacavir. AIDS. 2002. 16:2223–2225.

6. Hetherington S, Hughes AR, Mosteller M, Shortino D, Baker KL, Spreen W, Lai E, Davies K, Handley A, Dow DJ, Fling ME, Stocum M, Bowman C, Thurmond LM, Roses AD. Genetic variations in HLA-B region and hypersensitivity reactions to abacavir. Lancet. 2002. 359:1121–1122.

7. Hughes AR, Mosteller M, Bansal AT, Davies K, Haneline SA, Lai EH, Nangle K, Scott T, Spreen WR, Warren LL, Roses AD. CNA30027 Study Team. CNA30032 Study Team. Association of genetic variations in HLA-B region with hypersensitivity to abacavir in some, but not all, populations. Pharmacogenomics. 2004. 5:203–211.

8. Cao K, Hollenbach J, Shi X, Shi W, Chopek M, Fernández-Viña MA. Analysis of the frequencies of HLA-A, B, and C alleles and haplotypes in the five major ethnic groups of the United States reveals high levels of diversity in these loci and contrasting distribution patterns in these populations. Hum Immunol. 2001. 62:1009–1030.

9. Sun HY, Hung CC, Lin PH, Chang SF, Yang CY, Chang SY, Chang SC. Incidence of abacavir hypersensitivity and its relationship with HLA-B*5701 in HIV-infected patients in Taiwan. J Antimicrob Chemother. 2007. 60:599–604.

10. Lee KW, Oh DH, Lee C. Allelic and haplotypic diversity of HLA-A,-B,-C,-DRB1, and -DQB1 genes in the Korean population. Tissue Antigens. 2005. 65:437–447.

11. Park WB, Choe PG, Song KH, Lee SW, Jang HC, Jeon JH, Park SW, Park MH, Oh MD, Choe KW. Should HLA-B*5701 screening be performed in every ethnic group before starting abacavir? Clin Infect Dis. 2009. 48:365–367.

12. The Korean Society for AIDS. Clinical guidelines for diagnosis and treatment of HIV/AIDS in HIV-infected Koreans. Infect Chemother. 2011. 43:89–128.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download