Abstract
In Korea, Plasmodium vivax (P. vivax) is the most common agent of malaria infection. However, as travel to regions where malaria is endemic increases, so do the numbers of Plasmodium falciparum and mixed infections. P. falciparum predominates, while P. vivax is rare in west-central Africa. We report on a case of mixed malaria infection with severe hemolytic anemia caused by P. falciparum and P. vivax in a 38-year-old man after traveling to Angola. A diagnosis of P. falciparum malaria was made by microscopic examination. However, both P. vivax and P. falciparum were detected by the polymerase chain reaction (PCR). As a radical cure P. vivax, the patient was treated with mefloquine, artemether, and primaquine. Both P. falciparum and P. vivax had disappeared from peripheral blood by admission day 4, however, low grade fever and headache persisted, and his hemoglobin and hematocrit levels were depleted. A peripheral blood smear was negative for both P. vivax and P. falciparum; however, a direct anti-globulin test and anti-nuclear antibody test were positive, suggesting immune hemolytic anemia. After conservative treatment, which included a transfusion with packed red blood cells (RBC), his symptoms and signs showed improvement and laboratory findings were normalized.
Four Plasmodium species are generally infectious to humans, notably Plasmodium falciparum (P. falciparum), P. vivax, P. malariae, and P. ovale, as well as P. knowles, in rare cases. In South Korea, approximately 1,000 cases of malaria are reported annually [1]. However, in parallel with travel abroad, the number of patients infected with P. falciparum is increasing. In South Korea, the majority of malaria cases are caused by a single species. Mixed malaria infection is rare in Korea, and all reported cases have been imported [1]. In west-central Africa, P. falciparum is predominant and P. vivax is rare. And mixed infection with P. falciparum and P. vivax is also rare.
We report on a case of mixed infection with severe immune hemolytic anemia caused by P. falciparum and P. vivax after travel to Angola.
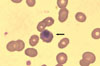
A 36-year old male patient visited the hospital with a three-day history of nausea, headache, abdominal pain, and fever for one day. Before admission to the hospital, he had traveled to Luanda and Angola for one month. Before going to Angola, he received a vaccine for yellow fever, but did not take prophylactic medication for malaria. On physical examination, he had no anemic conjunctiva, hepatosplenomegaly, body temperature 38.1℃, heart rate 113/min, respiration rate 22/min, and blood pressure 108/62 mmHg. He had been in good health. On admission, laboratory findings were as follows; platelet 220×103/mm3, hemoglobin 13.7 g/dL, white blood cell 3.3×103/mm3 (neutrophil 90%, lymphocyte 8%, monocyte 2%), AST/ALT 79/99 IU/L, total bilirubin 1.16 mg/dL, and Blood urea nitrogen (BUN)/Creatinine 6.0/0.9 mg/dL. Peripheral blood smear revealed that red blood cells (RBC) were normal in size, with a multiple ring form, and Mauer's dots, and parasite density was 3.5% (Fig. 1). Polymerase chain reaction (PCR) was performed for identification of Plasmodium species. Anti-malarial chemotherapy using artemeter 220 mg (3.2 mg/kg) was administered on the first day of treatment, and then, 110 mg (1.6 mg/kg) per day for four days, followed by mefloquine 1,250 mg (750 mg, then 500 mg 12 hr later). After anti-malarial treatment, parasite density decreased to 3.2% on day 1, 0.5% on day 2, and 0% on day 3. However, on day 4, the patient developed a single spiking fever up to 38.4℃ with abdominal discomfort. Abdominal computerized tomography was performed, but revealed only hepatosplenomegaly. On day 10, Plasmodium PCR revealed mixed infection of P. vivax and P. falciparum (Fig. 2). A 14-day schedule of primaquine 15 mg/day was started for radical cure of P. vivax. On day 11, hemoglobin had decreased to 7.9 g/dL, and the patient was discharged against advice. On day 14, he was re-admitted because of mild fever, dizziness, and abdominal pain. On physical examination, he had anemic conjunctiva, hepatosplenomegaly, body temperature 36.4℃, heart rate 72/min, respiration rate 18/min, and blood pressure 130/80 mmHg.
Laboratory findings at the second admission were as follows: Hb 5.9 g/dL, WBC 7.0×103/mm3 (neutrophil 53%, lymphocyte 36%, monocyte 11%), platelet 223×103/mm3, AST/ALT 43/23 IU/L, lactate dehydrogenase (LDH) 3,967 IU/L, total bilirubin 2.39 mg/dL, direct bilirubin 0.6 mg/dL, BUN 13.2 mg/dL, creatinine 0.8 mg/dL, proteinuria (+), microscopic hematuria (+), and urobilinogen (+). No malarial parasite was found in the peripheral blood smear (Fig. 3). Several tests were performed in order to evaluate the cause of aggravating anemia. Among those, the result of the direct anti-globulin test was positive for C3d type, indirect anti-globulin test was negative, haptoglobin 2.1 mg/dL and antinuclear antibody (ANA) was positive (titer 1:160), showing a nucleolar pattern. Immune hemolytic anemia was suggested as a cause of anemia and conservative care, including transfusion of packed RBC, was administered. His symptoms, including fever, showed improvement, and his hemoglobin had recovered up to 9.9 g/dL before discharge from the hospital. At the outpatient clinic, the patient was fully recovered.
Prevalence of mixed infection is relatively common in areas endemic for multiple malaria species, including Africa, east-south Asia, and Papua New Guinea [2-5]. In addition, travelers to these areas also have a relatively high prevalence of mixed infection. For example, 5% of infections in US Marines returning from Somalia were of mixed types [6]. However, the prevalence of mixed infection in Angola is generally low [7]. And the prevalence of P. vivax is also low in Angola. In Angola, In Angola, P. falciparum is observed in more than 92% of malaria patients, and P. vivax is observed in only 5-7% of malaria patients.
This low prevalence represents the high prevalence of the Duffy antigen-negative phenotype in these provinces, compared to other provinces and countries. Duffy antigen is the receptor for the plasmodial attachment to the red blood cell [8]. However, recent studies have reported that Duffy negative people could be infected with P. vivax [9]. Receptors other than Duffy antigen are considered as the cause of P. vivax infection in Duffy negative people [9].
The 10 cases of mixed infection reported in Korea for the years 2002 to 2008 represented 4% of the cases of imported malaria [1]. P. falciparum with P. vivax were the species found in all of these mixed infections [1]. Asia was the continent where mixed infection occurred most commonly (five of 10 cases), followed by Africa (three of 10 cases) [1]. Since 2002, three cases of mixed malaria infection have been reported in Korea [10-12]. Three of these patients had traveled to Asia (Malaysia, Cambodia, and Papua New Guinea); two patients had cerebral malaria and one patient died. Contrary to previously reported cases involving mixed infection, our patient was not a relapsed case of malaria, but a case of progressive worsening, which was initially diagnosed as mono-species infection (P. falciparum).
Mixed infection may result from simultaneous inoculation of sporozoites from multiply infected mosquitoes. However, separate inoculation of sporozoites from mosquitoes infected with a single species is a major route of mixed infection. Entomological studies have reported a relatively low rate of mixed infection in mosquitoes, compared to the rate of human mixed infection. In Papua New Guinea, for example, only four of 175 infected mosquitoes were found to carry mixed malarial organisms (P. falciparum with P. vivax) [13], while the prevalence of mixed infection was relatively high (113 of 163) in patients [2]. In Angola, the rate of mixed infection in human beings is low (four of 245 patients), but the rate of mixed infection in mosquitoes is unknown. However, in Equatorial Guinea (neighboring country of Angola), the rate of mixed infection in human beings is higher than that in mosquitoes [9].
Clinical manifestations of mixed and mono-species infections are similar; however, prognosis for a mixed infection is better than that for a mono-species one. Some studies have reported a lower rate of severe disease in infection with both P. falciparum and P. vivax than in infection with P. falciparum only. Clinical symptoms such as anemia, fever, and parasitemia may occur less frequently or to lower degrees in mixed infection than in monospecies infection [14]. However, contradictory findings have also been reported [5].
The pathogenesis of anemia in malaria is complex; possible mechanisms include direct destruction of infected RBC, complement-mediated lysis of uninfected RBC, phagocytosis of uninfected RBC by spleen, retention of blood in spleen, bone marrow suppression, and inefficient erythropoiesis. Of these mechanisms, direct lysis of infected RBC plays a lesser role than immunologic processes, which have been shown to cause hemolytic anemia [15]. In one study, the prevalence of direct antiglobulin test (DAT) positivity was 56% and this finding showed an association with anemia [16]. Our patient was positive for DAT, the C3d fragment of C3 complement, and hemolytic anemia. We concluded that the severe anemia in our patient was caused by immune-mediated hemolysis.
Our patient also showed a nucleolar pattern of ANA staining, with an ANA titer of 1:160. Positivity for ANA has been reported in 11% to 89% of malaria patients [17]. Such laboratory findings suggest an association of the immunological response to malaria with development of rheumatologic disease. Therefore, some researchers recommend follow-up serologic tests, such as ANA, or clinical examination for symptoms of rheumatologic disease [18].
Accurate diagnosis of malaria is important for appropriate management of the disease. Microscopic examination of a peripheral blood smear is the standard diagnostic method. When parasitemia occurs, microscopic examination is highly sensitive in identification of malaria species. A thick blood film provides greater sensitivity than a thin blood film, but requires more experience and skill in examination. Therefore, a thin blood film is used routinely for detection and counting of parasites. Rapid diagnostic tests (RDTs), such as malarial antigen or antibody detection using immunochromatographic assays (ICA) present alternatives to microscopic examination. Automated blood cell analysis, immunoassays, fluorescence microscopy, and molecular methods for malaria diagnosis are also in development. ICA methods are used most commonly in many endemic and non-endemic areas. The target antigens used are histidine-rich protein 2 (HRP-2) or lactate dehydrogenase (LDH), which are specific to P. falciparum, and aldolase or Plasmodium LDH, which are common malarial antigens. The performance of RDTs with ICA is generally acceptable for clinical use. The overall sensitivity of RDTs for P. falciparum and P. vivax has been reported as 90% [19]. However, at low levels of parasitemia, the sensitivity and specificity of RDTs are lower than at high levels of parasitemia, and these tests provide limited information on the positive or negative status of malaria, regardless of species. Thus, RDTs alone cannot accurately identify the infecting species or detect a mixed infection.
Molecular tests provide the most sensitive and conclusive tool for use in diagnosis of malaria, and can identify the infecting species and the presence of mixed infection. Amplification of the 18S ribosomal RNA (rRNA) yields different products according to the species present. The prevalence of malarial infection and mixed infection as evaluated using molecular tests is higher than the prevalence determined using microscopic examination or RDTs. Therefore, in patients suspected as having malaria, despite repeated negative microscopic examinations, molecular tests should be used for monitoring of treatment response, identification of species, and achievement of diagnosis. In the case of imported malaria, molecular tests are recommended in order to rule out mixed infection and to identify P. falciparum [20]. In our patient, based on microscopic examination, we initially suspected P. falciparum, however, results of PCR indicated a mixed infection.
In conclusion, the patient with imported malaria should undergo testing in order to exclude the possibility of mixed infection, which may lead to lethal complications, such as hemolytic anemia and splenomegaly. A mixed infection may be difficult to detect using microscopic examination or/and RDTs; therefore, molecular tests may be required. Because the risk of relapse is high, follow-up tests, such as the peripheral blood smear examination or complete blood cell count, are recommended.
Figures and Tables
 | Figure 1Peripheral blood smears at first admission (Wright-Giemsa stain, ×400). (A) A ring-form of malaria parasites (marginal or appliqué appearance) with Maurer's dot indicates P. falciparum. (B) Two-ring form of malaria parasites are observed in one red blood cell. |
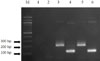 | Figure 2Multiplex PCR for detection of malaria. The band of 205-bp indicates P. falciparum. The band of 120-bp indicates P. vivax. lane M, size marker; lane 1, negative control for P. falciparum; lane 2, negative control for P. vivax; lane 3, positive control for P. falciparum; lane 4, positive control for P. vivax; lane 5, patient specimen with primer for P. falciparum; lane 6, patient specimen with the primer for P. vivax. |
References
1. Korea Center for disease control & prevention. 2009 Malaria. Accessed 11 February 2012. Available at: http://www.cdc.go.kr.
2. Mehlotra RK, Lorry K, Kastens W, Miller SM, Alpers MP, Bockarie M, Kazura JW, Zimmerman PA. Random distribution of mixed species malaria infections in Papua New Guinea. Am J Trop Med Hyg. 2000. 62:225–231.

3. Nathwani D, Badial R, Khaund RR, Douglas JG, Smith CC. Malaria in Aberdeen: an audit of 110 patients admitted between 1980-1991. Scott Med J. 1992. 37:106–110.

4. Krudsood S, Wilairatana P, Mason DP, Treeprasertsuk S, Singhasivanon P, Looareesuwan S. Hidden Plasmodium falciparum infections. Southeast Asian J Trop Med Public Health. 1999. 30:623–624.
5. Lyn PC. Cerebral malaria and mixed falciparum-vivax infections. Ann Acad Med Singapore. 1987. 16:310–312.
6. Newton JA Jr, Schnepf GA, Wallace MR, Lobel HO, Kennedy CA, Oldfield EC 3rd. Malaria in US Marines returning from Somalia. JAMA. 1994. 272:397–399.

7. Inquérito de Indicadores de Malária em Angola 2011. Accessed 11 February 2012. Available at: http://www.measuredhs.com.
8. Miller LH, Mason SJ, Dvorak JA, McGinniss MH, Rothman IK. Erythrocyte receptors for (Plasmodium knowlesi) malaria: Duffy blood group determinants. Science. 1975. 189:561–563.

9. Mendes C, Dias F, Figueiredo J, Mora VG, Cano J, de Sousa B, do Rosário VE, Benito A, Berzosa P, Arez AP. Duffy negative antigen is no longer a barrier to Plasmodium vivax--molecular evidences from the African West Coast (Angola and Equatorial Guinea). PLoS Negl Trop Dis. 2011. 5:e1192. Epub 2011 Jun 21.
10. Shin KS, Kim JS, Ryu SW, Suh IB, Lim CS. A case of Plasmodium vivax infection diagnosed after treatment of imported falciparum malaria. Korean J Clin Pathol. 2001. 21:360–364.
11. Jeong AC, Ahn BJ, Choi CK, Yoon KS, Nam HW, Lee WJ, Lee JS. A case of mixed malarial infection wtih Plasmodium falciparum and Plasmodium vivax. Korean J Infect Dis. 1998. 30:194–197.
12. Kim JM, Yoo TH, Park CJ, Chi HS. A mixed cerebral infection of vivax and falciparum malaria. Korean J Clin Pathol. 2000. 20:263–267.
13. Cooper RD, Waterson DG, Frances SP, Beebe NW, Pluess B, Sweeney AW. Malaria vectors of Papua New Guinea. Int J Parasitol. 2009. 39:1495–1501.

14. Alifrangis M, Lemnge MM, Moon R, Theisen M, Bygbjerg I, Ridley RG, Jakobsen PH. IgG reactivities against recombinant Rhoptry-Associated Protein-1 (rRAP-1) are associated with mixed Plasmodium infections and protection against disease in Tanzanian children. Parasitology. 1999. 119(Pt 4):337–342.

15. Drouin J, Rock G, Jolly EE. Plasmodium falciparum malaria mimicking autoimmune hemolytic anemia during pregnancy. Can Med Assoc J. 1985. 132:265–267.
16. Facer CA, Bray RS, Brown J. Direct Coombs antiglobulin reactions in Gambian children with Plasmodium falciparum malaria. I. Incidence and class specificity. Clin Exp Immunol. 1979. 35:119–127.
17. Daniel Ribeiro CT, de Roquefeuil S, Druilhe P, Monjour L, Homberg JC, Gentilini M. Abnormal anti-single stranded (ss) DNA activity in sera from Plasmodium falciparum infected individuals. Trans R Soc Trop Med Hyg. 1984. 78:742–746.

18. Cho SE, Huh JW, Chung WS. Antinuclear Antibody Production in Patients with Malaria Infection. Korean J Clin Pathol. 2001. 21:385–389.
19. Ashley EA, Touabi M, Ahrer M, Hutagalung R, Htun K, Luchavez J, Dureza C, Proux S, Leimanis M, Lwin MM, Koscalova A, Comte E, Hamade P, Page AL, Nosten F, Guerin PJ. Evaluation of three parasite lactate dehydrogenase-based rapid diagnostic tests for the diagnosis of falciparum and vivax malaria. Malar J. 2009. 8:241.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download