Abstract
Erythema elevatum diutinum (EED) is emerging as a specific HIV-associated dermatosis which can be easily misdiagnosed as Kaposi's sarcoma or bacillary angiomatosis. Until now, no case of HIV-associated EED had been reported in Korea. We report a case of EED in a 49-year-old man with HIV infection. The patient was diagnosed with HIV-infection and treated with a combination of anti-retroviral agents and dapsone. Two years after the start of treatment the lesion had regressed.
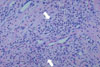
Erythema elevatum diutinum (EED) is an uncommon chronic dermatosis which is clinically characterized by violaceous papules, plaques and nodules that are typically distributed acrally and symmetrically over extensor surfaces [1]. EED is known to be associated with repetitive bacterial infections (particularly streptococcal), monoclonal gammopathy and connective tissue disease. Recently, an association of EED with HIV infection had been reported [2]. EED is diagnosed by the use of a lesion biopsy with histological findings including leukocytoclastic vasculitis, perivascular infiltrate (consisting mainly of polymorphonuclear neutrophils), nuclear dust, and to a lesser extent macrophages, lymphocytes and eosinophils [3]. In the later phase of the progression of EED, granulation and fibrotic tissue are dominant and cholesterol crystals may be observed intra/extracellularly as a secondary change [3].
Even though its association with HIV infection is infrequent, EED is emerging as a specific HIV-associated dermatosis, and it is easily misdiagnosed as Kaposi's sarcoma or bacillary angiomatosis [4, 5]. Because of the peculiarities of the disease presentation the correct diagnosis of EED in HIV-infected patients may be difficult [5]. EED had not been previously reported in Korea but we now describe the first Korean case of a man diagnosed with HIV for whom EED appeared as the first clinical manifestation. He was treated with antiretroviral agents and dapsone.
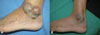
A 49-year-old man presented with masses growing on both the right and left external sides of his ankles since one and half years. The patient had been treated for herpes zoster infection in the chest region twice, at 1 and 5 years prior to his visit. At admission, he had a blood pressure of 120/80 mmHg, pulse rate of 80/min, respiration rate of 18/min and body temperature of 36.4℃. He presented with numerous clustered vesicles and macular lesions, with erythema and pain on the left arm and back of the hand, clinical signs which suggested herpes zoster infection. Numerous purpuric lesions were observed on both of the patient's legs. On the exterior side of the left ankle, multiple nodular masses of variable sizes were observed and the largest mass was about 5 cm in diameter and included a central ulcer (Fig. 1A). On the lateral malleolar region of the right ankle, similar masses of smaller sizes were observed. There was no tenderness found upon palpation of the masses on either leg. His blood and clinical chemistry test results were as follows: white blood cells; 4,730/mm3 (neutrophils 77% and lymphocytes 14%), hemoglobin; 15.7 g/dL, platelet; 25,000/mm3, AST; 41 IU/L, ALT; 79 IU/L, total protein; 7.3 g/dL, albumin; 4.0 g/dL, BUN; 16.0 mg/dL and creatinine; 0.8 mg/dL. His serology result was negative for anti-HBV and anti-HCV antibodies. Anti-HIV-antibody was positive 15.4/uL of CD4+ T cells and 2.83×105 copies/mL of HIV-RNA. A resection biopsy was taken of the mass on the left ankle and the histologic findings were compatible with EED (Fig. 2).
He was treated with daily doses of atazanavir of 300 mg, ritonavir of 100 mg, zidovudine of 600 mg, lamivudine of 300 mg and dapsone of 100 mg. Prophylatic trimethoprim/sulfamethoxazole was also maintained. Two months after initiation of treatment his platelet count had increased to 121,000/mm3. After two years of treatment with a combination of anti-retroviral agents and dapsone, his CD4+ T cell count had increased to 162/uL and his HIV-RNA results decreased to less than 20 copies/mL. The lesions had regressed and he did not feel any discomfort (Fig. 1B).
The onset of initial EED lesions may be associated with pruritus or a burning sensation. Established lesions may be tender, although some cases are asymptomatic. EED is observed most commonly in patients in the fourth through sixth decades of life and with a slightly male predominance [6]. Controversy exists regarding the etiology of EED. Nevertheless, the most widely accepted theory is that previous and repeated exposure to bacterial infections, particularly those of streptococcal origin, may trigger an immunological reaction that culminates in an outbreak of skin lesions [1, 7]. Improvement of symptoms may result by treatment of an underlying cause or infection [8]. EED has been reported to occur in conjunction with various hematological abnormalities including myelodysplasia, myeloproliferative alterations, multiple myeloma [3, 9], cryoglobulinemia [10], rheumatic arthritis [3, 11, 12], prostate carcinoma [3, 13], testicular lymphoma [3], celiac disease [14], Crohn's disease [15] and relapsing polychondritis [16].
To the best of our knowledge, fewer than 20 cases of HIV-associated EED have been reported worldwide. Even though the association of EED with HIV infection is infrequent, laboratory investigation for HIV should be conducted in conventional cases, and especially for cases with atypical and exacerbated clinical manifestations [2]. The EED lesions associated with HIV infection have been described as nodular with palmar/plantar involvement with greater numbers of lesions present at an earlier age. As in this present case, the existence of a nodular form of EED is highly suggestive of an underlying HIV infection [5, 18, 19]. Therefore, a patient with a nodular form of EED must be evaluated for HIV. EED is also easily misdiagnosed as Kaposi's sarcoma or bacillary angiomatosis, but the histopathological features are diagnostic [2, 4, 5]. Histopathological evaluation of the lesion is essential for differentiating EED from Kaposi's sarcoma or bacillary angiomatosis. In this present case, Kaposi's sarcoma was highly suspected (Fig. 1A) but the histopathological findings revealed EED.
Dapsone and sulfonamides are considered first-line treatments for EED, although lesions often recur with cessation of these therapies [17]. However, dapsone may be less effective in lesions that have progressed to the more fibrotic stage. Surgical excision of larger nodules has also been performed with some success [5]. While HIV-associated EED is generally known to be unresponsive to dapsone [17, 19], as in the present case, some cases of EED associated with HIV infection have demonstrated a good response to dapsone alone or in combination with antiretroviral therapy [8, 18]. Dapsone is an alternative prophylactic regimen for Pneumocystis jiroveci infection, but this regimen does not prevent toxoplasmosis. Thus in this case, prophylactic administration of trimethoprim/sulfamethoxazole was maintained along with dapsone. One month after initiation of the antiretroviral therapy, the CD4+ T lymphocyte count rose above 100/uL. Therefore, prophylaxis for Mycobacterium avium complex was not provided.
Considering these results, EED should be included in the differential diagnosis of skin lesions affecting HIV positive patients. We report the first Korean case of erythema elevatum diutinum (EED), which presented as the initial manifestation of HIV infection, and was subsequently treated by antiretroviral agents with dapsone.
Figures and Tables
References
1. Katz SI, Gallin JI, Hertz KC, Fauci AS, Lawley TJ. Erythema elevatum diutinum: skin and systemic manifestations, immunologic studies, and successful treatment with dapsone. Medicine (Baltimore). 1977. 56:443–455.

2. Rover PA, Bittencourt C, Discacciati MP, Zaniboni MC, Arruda LH, Cintra ML. Erythema elevatum diutinum as a first clinical manifestation for diagnosing HIV infection: case history. Sao Paulo Med J. 2005. 123:201–203.

3. Yiannias JA, el-Azhary RA, Gibson LE. Erythema elevatum diutinum: a clinical and histopathologic study of 13 patients. J Am Acad Dermatol. 1992. 26:38–44.

4. Requena L, Sánchez Yus E, Martín L, Barat A, Arias D. Erythema elevatum diutinum in a patient with acquired immunodeficiency syndrome. Another clinical simulator of Kaposi's sarcoma. Arch Dermatol. 1991. 127:1819–1822.

5. Muratori S, Carrera C, Gorani A, Alessi E. Erythema elevatum diutinum and HIV infection: a report of five cases. Br J Dermatol. 1999. 141:335–338.

7. Kohler IK, Lorincz AL. Erythema elevatum diutinum treated with niacinamide and tetracycline. Arch Dermatol. 1980. 116:693–695.

8. Suárez J, Miguélez M, Villalba R. Nodular erythema elevatum diutinum in an HIV-1 infected woman: response to dapsone and antiretroviral therapy. Br J Dermatol. 1998. 138:717–718.

9. Wilkinson SM, English JS, Smith NP, Wilson-Jones E, Winkelmann RK. Erythema elevatum diutinum: a clinicopathological study. Clin Exp Dermatol. 1992. 17:87–93.

10. Morrison JG, Hull PR, Fourie E. Erythema elevatum diutinum, cryoglobulinaemia, and fixed urticaria on cooling. Br J Dermatol. 1977. 97:99–104.

11. Nakajima H, Ikeda M, Yamamoto Y, Kodama H. Erythema elevatum diutinum complicated by rheumatoid arthritis. J Dermatol. 1999. 26:452–456.

12. Collier PM, Neill SM, Branfoot AC, Staughton RC. Erythema elevatum diutinum--a solitary lesion in a patient with rheumatoid arthritis. Clin Exp Dermatol. 1990. 15:394–395.

13. Henriksson R, Hofer PA, Hörnqvist R. Erythema elevatum diutinum--a case successfully treated with colchicines. Clin Exp Dermatol. 1989. 14:451–453.

14. Tasanen K, Raudasoja R, Kallioinen M, Ranki A. Erythema elevatum diutinum in association with coeliac disease. Br J Dermatol. 1997. 136:624–627.

15. Walker KD, Badame AJ. Erythema elevatum diutinum in a patient with Crohn's disease. J Am Acad Dermatol. 1990. 22:948–952.

16. Bernard P, Bedane C, Delrous JL, Catanzano G, Bonnetblanc JM. Erythema elevatum diutinum in a patient with relapsing polychondritis. J Am Acad Dermatol. 1992. 26:312–315.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download