Abstract
We describe a case of idiopathic CD4+ T-lymphocytopenia (ICL) in a 59-year-old patient who presented with various opportunistic infections. The patient was diagnosed with disseminated Mycobacterium avium infection, cytomegalovirus colitis and retinitis, and esophageal candidiasis. He was successfully treated with anti-mycobacterial drugs, ganciclovir, and fluconazole, respectively. However, the patient was diagnosed with primary central nervous system lymphoma, and then died of a Trichosporon beigelii sepsis during the 2nd cycle of systemic chemotherapy.
It is widely accepted that idiopathic CD4+ T-lymphocytopenia (ICL) is a rare heterogeneous syndrome that is not caused by human immunodeficiency virus 1 (HIV-1), HIV-2, human T-cell lymphotropic virus I (HTLV-I), or HTLV-II, and does not appear to be caused by any transmissible agent[1]. The Centers for Disease Control and Prevention defines this condition as a CD4+ cell count <300 cells/mm3 or <20% of the total number of T-cells on more than one occasion, along with no evidence of HIV infection and the absence of any defined immunodeficiency or therapy that could depress CD4+ T-cell levels[2]. Patients with ICL have a variety of clinical spectrum, ranging from an asymptomatic course or minimal symptoms to death due to various opportunistic infections (OIs) or malignancies that are similar to those presented in HIV-infected patients[3]. In this report, we describe the first case of primary central nervous system lymphoma (PCNSL) affecting parenchymal brain tissue along with a variety of OIs in the context of ICL.
A 59-year-old man came to our hospital with the chief complaint of prolonged fever and watery diarrhea with no identified cause of disease. He had been in good health and had never traveled abroad or abused drugs. In addition, the patient had no history of blood transfusion, homosexuality, or any medical illness.
Upon admission, the patient appeared generally ill and cachectic. His vital signs were as follows: blood pressure was 107/60 mmHg, pulse rate 84 beats/min, respiratory rate 19 breaths/min, and axillary body temperature 38.0℃. Upon physical examination, there was no skin lesion, lymphadenopathy, or hepatosplenomegaly. Laboratory studies showed white blood cells 1,230/mm3 (segmented neutrophils 72%, lymphocytes 7%, monocytes 15%, eosinophils 6%), hemoglobin 11.3 g/dL, platelets 64×103/mm3, blood glucose 105 mg/dL, total bilirubin 0.8 mg/dL, aspartate aminotransferase (AST) 68 IU/L, alanin aminotransferase (ALT) 33 IU/L, lactate dehydrogenase (LDH) 410 IU/L, and C-reactive protein 5.28 mg/dL.
Chest computed tomography (CT), obtained with radiocontrast dye, showed multifocal ground glass opacities along with nodular consolidations in both lungs and abdomen and pelvic CT showed splenomegaly, heterogeneous liver attenuation, and enlargement of multiple mesenteric lymph nodes.
Meanwhile, colonoscopic examination revealed numerous aphthous ulcers with reddish mucosal changes from the rectum to transverse colon. Histologic examination showed CMV (cytomegalovirus) colitis. Upon ophthalmologic evaluation, small, opaque, and white areas of granular retinal necrosis with hemorrhage were observed in both retinas, leading to a presumptive diagnosis of CMV retinitis. Bone marrow examination was performed to evaluate the cause of prolonged fever and peripheral blood cytopenia. The results showed that cellularity was 11-20%, which was hypocellular for the patient' s age, and granulopoiesis, erythropoiesis, and megakaryocytes all decreased. A few noncaseating granulomas and acid-fast bacilli were observed, but no fungal element or evidence of other microorganisms and malignancies were observed. Bone marrow aspiration and sputum culture both grew mycobacterium after 3 and 6 weeks of incubation, respectively. All isolates were identified as Mycobacterium avium by means of polymerase chain reaction-restriction fragment length polymorphism analysis[4].
Although the patient's CD4+ cell count significantly decreased (CD4: 6 cells/mm3 with 10% of lymphocytes, CD8: 50 cells/mm3 with 81% of lymphocytes, and CD4/CD8 ratio: 0.12), test for HIV antibodies was negative according to both microparticle enzyme immunoassay (The AxSYM® HIV Ag/Ab Combo, Abbott, IL, USA) and chemiluminescent microparticle immunoassay (The Architect® HIV Ag/Ab Combo, Abbott, IL, USA), as was polymerase chain reaction (PCR) for HIV (Roche Amplicor HIV-1 monitor Test, Version 1.5). Test for antibodies to human T-cell leukemia virus (HTLV) was also negative. Serum immunoglobulin levels were IgG, 807 mg/dL (normal 700-1700); IgA, 49 mg/dL (normal 90-400); IgM, 30 mg/dL (normal 45-230).
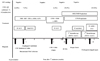
The patient was initially treated with a four-drug anti-tuberculosis regimen. Following the identification of pathogen, clarithromycin and ethambutol were used. Concomitantly diagnosed CMV colitis and CMV retinitis were treated with a 21-day induction course of ganciclovir, followed by a maintenance regimen of ganciclovir (5 mg/kg/d) for 5 days per week for 2 months. The fever dissipated and the diarrhea was resolved quickly, along with the gradual disappearance of chest infiltrations (Fig. 1).
Three weeks after admission, he complained of odynophagia and epigastric pain. Endoscopic examination of the upper digestive tract showed esophageal mucosa to be covered with whitish plaque-like lesions. This finding, together with his symptoms, led to a presumptive diagnosis of esophageal candidiasis, and he was treated accordingly with fluconazole. The patient's symptoms were alleviated after 2 weeks of treatment with fluconazole, and the plaque-like lesions of the esophagus also disappeared. He was discharged from the hospital 6 weeks after admission.
Two months after discharge, he was readmitted with diarrhea and abdominal pain. On the basis of the patient's clinical course and the positive endoscopic findings, a diagnosis of relapse of CMV colitis was made, and he was treated accordingly with a 21-day induction course of ganciclovir. Following this, the patient remained in good health while taking medications for prophylaxis of Mycobacterium avium complex (MAC) and Pneumocystis jirovecii.
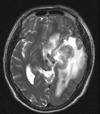
One and a half years after his first admission, the patient presented with the chief complaint of inappropriate speech with progressive disorientation over 1 month. Magnetic resonance imaging (MRI) of the brain revealed 3.5 cm sized lobulated lesions in the left basal ganglia and temporal lobe with extensive edema, which showed ring enhancement on contrast administration (Fig. 2). Stereotactic biopsy of the brain demonstrated diffuse large B-cell lymphoma with overexpression of p53 and high Ki-67 proliferative index over 95% positivity. The cerebral spinal fluid (CSF) was negative for malignant cells, and CT scans of the chest and abdomen were unremarkable. He was started on a chemotherapy regimen of methotrexate and cytarabine, resulting in prompt clinical improvement, with a reduction on subsequent MRI. However, his hospital course was further complicated by dermatomal zoster infection, although quickly resolved after treatment with acyclovir.
Two months after PCNSL diagnosis, the patient had a Trichosporon beigelii sepsis during the second round of chemotherapy. Despite therapy with amphotericin B and fluconazole, he died of sepsis. During the whole clinical course, the results of HIV serology were repeatedly negative and the patient's CD4+ T-lymphocyte count was persistently below 300 cells/mm3.
The hallmark of ICL is continued severe depletion of CD4+ T-lymphocytes, not attributable to HIV, HTLV, immunosuppressive medications, or other immune disorders. We believe that the condition of our patient meets the diagnostic criteria for ICL as he had no evidence of HIV infection and had never received immunosuppressive drugs and his CD4+ T-lymphocyte count was below 300 cells/mm3 during the long follow-up period. Further the low levels of serum immunoglobulins in our patient are characteristic of ICL, unlike those in patients with HIV infection[5].
Investigations into potential cases of idiopathic depletion of CD4+ T-lymphocyte have found that true cases are very rare. Most of these cases actually represent other disorders and a low CD4+ T-lymphocyte count may reflect a transient response to infection or even normal findings in asymptomatic patients. The temporal relationship between mycobacterial infections, P. jirovecii pneumonia, extrapulmonary fungal and parasitic infections, and a suppressed CD4+ T-lymphocyte count is unclear. Common pathogenic and opportunistic bacteria, viral, parasitic, and fungal infections may depress CD4+ cell counts, which change is mostly transient without inversion of the CD4 : CD8 ratio[6]. In particular, disseminated and severe tuberculosis is well-known to be a reversible cause of CD4+ T-lymphocytopenia in HIV-seronegative individuals[7]. In our case, however, after complete resolution of multiple episodes of OIs, profound depletion of CD4+ lymphocytes was persistently observed, which suggests that OIs alone, including disseminated Mycobacterium avium infection, cannot be the sole cause.
In most reported cases, patients with ICL present with OIs, including nontuberculous mycobacterial infection, cryptocooccal meningitis, P. jirovecii pneumonia, oral candidiasis, cutaneous herpes zoster, human papilloma virus (HPV) infections, and cerebral toxoplasmosis, although some patients do not have OIs and are relatively healthy at the time of diagnosis[8-10]. Still, the optimal treatment and prophylaxis for OIs in patients with ICL remain to be defined. CD4+ cell counts provide an effective surrogate marker for clinical disease progression in HIV-infected patients[11, 12]. However, it is unclear whether or not similar principles apply to patients with ICL. The occurrence of multiple OIs and relapses in individual patients as shown in our case suggests that an aggressive approach to diagnosis, treatment, and prophylaxis is needed.
The literature regarding ICL patients with non-Hodgkin's lymphoma (NHL) is limited to a small number of case reports , moreover, in most of which the brain was not involved[5, 13-16]. To our knowledge, this is the first reported case of PCNSL affecting parenchymal brain tissue in the context of ICL. It is well known that patients with depressed CD4+ T cell numbers secondary to HIV infection are at a greater risk for developing NHL[17, 18]. Conversely, lymphoproliferative disorders may cause CD4+ lymphocytopenia, either through trafficking and homing of lymphocytes to lymphoid organs or through an absolute quantitative deficiency[19]. According to the literature, there is no definite explanation regarding the sequential relationship between low CD4+ count and development of lymphoma. However, in the present case, PCNSL was diagnosed 1.5 years after the onset of CD4+ T-lymphocytopenia. Moreover, brain MRI performed 1 year ago before the onset of neurologic symptoms showed no abnormal findings in the brain tissue, which raises the possibility that CD4+ T-lymphocytopenia was a pre-existing condition rather than the result of malignancy. Other case reports on diffuse large B-cell lymphoma[14, 20] also bolster this idea, in that CD4+ T-lymphocytopenia preceded malignancy for several years and persisted after complete remission of lymphoma.
It is clear that idiopathic CD4+ T-lymphocytopenia should be included in the differential diagnosis of unexplained OIs. Immunodeficiency can exist in the absence of laboratory evidence of HIV infection, thus underscoring the importance of evaluating T-cell subsets in patients who present with unusual infections. Our case illustrates that various OIs and malignancy can develop simultaneously or sequentially in a patient with ICL and further highlights the need for further research into the pathogenesis and adequate treatment of ICL.
Figures and Tables
Figure 1
Patient's clinical course.
AMB, amphotericin B; AMK, amikacin; Ara-C, cytarabine; CMV, cytomegalovirus; CTM, clarithromycin; EMB, ethambutol; FCZ, fluconazole; GCV, ganciclovir; INH, isoniazid; LFX; levofloxacin; MTX, methotrexate; MAC, mycobacterium avium complex; PCNSL, primary central nervous system lymphoma; PZA, pyrazinamide; RFB, rifabutin; RIF, rifampin; SMX/TMP, sulfamethoxazole/Trimethoprim.
References
1. Fauci AS. CD4+ T-lymphocytopenia without HIV infection--no lights, no camera, just facts. N Engl J Med. 1993. 328:429–431.

2. Centers for Disease Control (CDC). Unexplained CD4+ T-lymphocyte depletion in persons without evident HIV infection--United States. MMWR Morb Mortal Wkly Rep. 1992. 41:541–545.
4. Park CM, Heo SR, Park KU, Song J, Lee JH, Lee CT, Kim EC. Isolation of nontuberculous mycobacteria using polymerase chain reaction-restriction fragment length polymorphism. Korean J Lab Med. 2006. 26:161–167.

5. Smith DK, Neal JJ, Holmberg SD. The Centers for Disease Control Idiopathic CD4+ T-lymphocytopenia Task Force. Unexplained opportunistic infections and CD4+ T-lymphocytopenia without HIV infection An investigation of cases in the United States. N Engl J Med. 1993. 328:373–379.

6. Laurence J. T-cell subsets in health, infectious disease, and idiopathic CD4+ T lymphocytopenia. Ann Intern Med. 1993. 119:55–62.

7. Kony SJ, Hane AA, Larouzé B, Samb A, Cissoko S, Sow PS, Sané M, Maynart M, Diouf G, Murray JF. Tuberculosis-associated severe CD4+ T-lymphocytopenia in HIV-seronegative patients from Dakar. SIDAK Research Group. J Infect. 2000. 41:167–171.

8. Zonios DI, Falloon J, Bennett JE, Shaw PA, Chaitt D, Baseler MW, Adelsberger JW, Metcalf JA, Polis MA, Kovacs SJ, Kovacs JA, Davey RT, Lane HC, Masur H, Sereti I. Idiopathic CD4+ lymphocytopenia: natural history and prognostic factors. Blood. 2008. 112:287–294.

9. DeHovitz JA, Feldman J, Landesman S. Idiopathic CD4+ Tlymphocytopenia. N Engl J Med. 1993. 329:1045–1046.

10. Ahn IS, Kim HG, Ryu JS, Kim L, Kwak SM, Lee HL, Yoon YH, Cho JH. A case of pulmonary cryptococcosis with non-small cell lung cancer in idiopathic CD4+T-lymphocytopenia. Yonsei Med J. 2005. 46:173–176.

11. Fahey JL, Taylor JM, Detels R, Hofmann B, Melmed R, Nishanian P, Giorgi JV. The prognostic value of cellular and serologic markers in infection with human immunodeficiency virus type 1. N Engl J Med. 1990. 322:166–172.

12. Stein DS, Korvick JA, Vermund SH. CD4+ lymphocyte cell enumeration for prediction of clinical course of human immunodeficiency virus disease: a review. J Infect Dis. 1992. 165:352–363.

13. Busse PJ, Cunningham-Rundles C. Primary leptomeningeal lymphoma in a patient with concomitant CD4+ lymphocytopenia. Ann Allergy Asthma Immunol. 2002. 88:339–342.

14. Campbell JK, Prince HM, Juneja SK, Seymour JF, Slavin M. Diffuse large cell lymphoma and t(8;22) (q24;q11) in a patient with idiopathic CD4+ T-lymphopenia. Leuk Lymphoma. 2001. 41:421–423.

15. Cook MA, Bareford D, Kumararatne DS. Non-Hodgkin's lymphoma: an unusual complication of idiopathic CD4+ lymphopenia. Hosp Med. 1998. 59:582.
16. Quiles I, Anaut P, Cibrián F, Gainzaráin J, Vega L, Andía A. Idiopathic CD4+ T-lymphocytopenia with opportunistic infection and non-Hodgkin's lymphoma. J Intern Med. 1995. 238:183–184.

17. Sewell HF, MacKenzie RH, Dawson AA, Ratcliffe MA, King DJ, Bennett NB. Phenotypic abnormality of T cells in B cell non-Hodgkin's lymphoma. Dis Markers. 1990. 8:145–149.
18. Beral V, Peterman T, Berkelman R, Jaffe H. AIDS-associated non-Hodgkin lymphoma. Lancet. 1991. 337:805–809.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download