Abstract
Clostridium tertium-induced bacteremia is a rare condition seen predominantly in neutropenic patients and/or patients with gastrointestinal disease. In this report, we describe a non-neutropenic, 72-year-old patient with a small bowel obstruction who presented with C. tertium bacteremia. Clostridium tertium is aerotolerant and resistant to broad-spectrum cephalosporins. The aerotolerant nature of C. tertium is resulted in delayed identification and reporting since it is not initially considered a candidate for infection.
The genus Clostridium is a diverse group of Gram-positive, spore-forming, anaerobic bacilli found in soil and the guts of many animals, including humans. Clostridium-induced bacteremia is a rare condition seen primarily in intra-abdominal infections associated with surgical trauma. It has also been associated with underlying gastrointestinal disorders, pregnancy, and malignancies [1].
Clostridium tertium was not recognized as a human pathogen until 1963 [2]. It is most frequently isolated from blood cultures, although it can also cause spontaneous bacterial peritonitis, brain abscesses, and pneumonia [3-5]. Most cases of C. tertium-induced bacteremia occur in neutropenic patients without a definite source of infection [3, 5]. Here, we describe a case of C. tertium bacteremia in a 72-year-old non-neutropenic patient with an intestinal obstruction.
A 72-year-old male was admitted with diffuse abdominal pain, which had started four days earlier. He had a history of colon cancer, diagnosed in 1998, and had been treated with a left hemicolectomy. Two years prior to admission, he was diagnosed with a hepatocellular carcinoma (B-viral) and had undergone five rounds of transcatheter arterial chemoembolization. Upon admission, his body temperature was 38.3℃, his heart rate was 93 beats per minute, his respiratory rate was 20 per min, and his blood pressure was 123/75 mmHg. Physical examination revealed increased bowel sounds and direct/rebound tenderness on the entire abdomen. His initial white blood cell count was 8,320/mm3 (86.5% neutrophils), his hemoglobin level was 12.6 g/dL, his platelet count was 6,800/mm3, and his C-reactive protein level was 246 mg/L. Abdominal CT revealed fluid-filled, dilated small bowel loops, most likely at the proximal ileum (Fig. 1).
With a preliminary diagnosis of small bowel obstruction, a nasogastric tube was inserted for decompression. Intravenous antibiotic therapy was initiated with piperacillin-tazobactam (4.5 g every 8 h). However, the small bowel obstruction did not improve with intravenous antibiotic therapy and supportive care. The patient had a persistent fever and severe abdominal pain. His blood leukocytes were also elevated (16,650/µL). These clinical findings, combined with a subsequent plain radiograph, led to suspicion of strangulation (Fig. 2)
On the fifth day after admission, laparotomy and segmental resection of the small bowel with end ileostomy were performed to confirm the strangulation. Tigecycline (50 mg every 12 h after an initial 100 mg dose) was administered intravenously due to a persistent fever, despite the use of piperacillin-tazobactam. On the seventh day after admission, a blood culture revealed C. tertium.
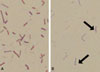
Upon admission, three sets of aerobic and anaerobic blood cultures were set up using the BacT/Alert 3D system (bioMérieux, Durham, NC, USA). The two anaerobic bottles yielded bacterial growth on the first day of incubation, while the corresponding aerobic bottles revealed growth three days later. Staining showed Gram-variable bacilli lacking spores. Sub-culturing from all of the positive bottles yielded colonies, but those on anaerobic plates were larger, suggesting that the organism was aerotolerant. Gram staining of the colonies from the anaerobic subculture revealed occasional terminal spores (Fig. 3). Bacterial identification using both the Rapid ID 32A system and ANI card (bioMérieux, Marcy l'Étoile, France) revealed C. tertium. The strain was confirmed by PCR and nucleotide sequencing of the bacterial 16S rRNA gene. The sequences were compared with known sequences in the EzTaxon server database (http://www.eztaxon.org; accessed 2 Mar 2011), which revealed 100% homology with C. tertium (GenBank accession number Y18174.1).
Three days post-operation, intra-abdominal bleeding was observed, and oliguric acute renal failure developed. Explorative laparotomy revealed active bleeding from the inferior epigastric artery in the left rectus muscle, and suture ligation was performed. In the presence of tigecycline, the same organism was observed at follow-up blood cultures on days 6 and 8, but not on day 13. The patient gradually deteriorated despite aggressive treatment and eventually died 19 days after admission due to multi-organ failure and acute respiratory distress syndrome.
Clostridium tertium bacteremia is a rare condition mainly associated with neutropenia in critically ill patients [6-8]. Miller et al. [3] reported that 91% (29/32) of cases of C. tertium-related bacteremia occurred in neutropenic patients. Several other studies have also reported neutropenia as a major factor in C. tertium bacteremia [6, 8-12]. The exact role of neutropenia in the pathogenesis of C. tertium is unclear. However, it may enable C. tertium to enter the systemic circulation and facilitate the organism' s growth by dampening the innate immune response [3].
In non-neutropenic patients, C. tertium bacteremia is associated with intestinal abnormalities. Three distinct cases of C. tertium bacteremia in non-neutropenic patients have been described [3]. Of these, one had severe liver disease and spontaneous bacterial peritonitis, one recently had a percutaneous gastrostomy tube implanted, and one had Crohn's disease [3]. A case of C. tertium bacteremia in non-neutropenic post-operative patients with a mechanical ileus has also been reported [13]. Clostridium tertium bacteremia in non-neutropenic patients is promoted by various abdominal disorders (e.g., chronic inflammatory bowel disease), as well as by the administration of anti-cancer drugs [7, 9]. In the current case, the patient was non-neutropenic but had a small bowel obstruction with strangulation. The obstruction damaged the intestinal mucosa, allowing C. tertium to enter the systemic circulation.
Clostridium tertium is not significantly pathogenic, and some authors claim that C. tertium is not a true pathogen, but merely a contaminant [14]. However, our patient was at risk (severe sepsis with intestinal obstruction) for C. tertium bacteremia, and C. tertium was isolated from all three blood cultures. Thus, C. tertium bacteremia, in this case, was not likely a result of culture contamination.
Clostridium tertium is not histotoxic, lipolytic, or toxin-producing. When C. tertium bacteremia is treated with appropriate antimicrobial therapies, the mortality rate is quite low [1]. In several studies, most patients with C. tertium bacteremia survived after appropriate antibiotic therapy [2, 3]. In this case, C. tertium did not grow in the blood cultures after the administration of tigecycline. The patient's cause of death was classified as multi-organ failure and acute respiratory distress syndrome due to post-operative bleeding.
Clostridium tertium is resistant to many beta-lactams, and it is often resistant to expanded-spectrum cephalosporins with enhanced activity against gram-positive bacteria [7]. A penicillin-resistant strain has also been reported [10]. Metronidazole, which is commonly used to treat anaerobic infections, may be effective [6, 10], but resistant C. tertium isolates have been described [11]. In vitro, tigecycline has been shown to have potent inhibitory activity against C. tertium [15].
Tigecycline was used to successfully treat patients with complicated intra-abdominal infections in several studies [16, 17]. Tigecycline is not significantly toxic and has a wide spectrum of antibiotic activities. We applied tigecycline as an alternative antimicrobial.
In conclusion, we describe a case of C. tertium bacteremia in a non-neutropenic patient with small bowel obstruction. Clostridium tertium rarely causes bacteremia in non-neutropenic patients. However, pre-existing intestinal mucosal injury increases susceptibility to C. tertium bacteremia. Furthermore, because of the aerotolerant nature of the isolate, Clostridium species were not initially considered, resulting in delayed identification and reporting.
Figures and Tables
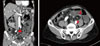 | Figure 1Abdominal computed tomography (CT) at admission shows fluid-filled, dilated small bowel loops at the proximal ileum. An adhesion band is a possible cause of the obstruction (red arrows). |
References
1. Allen SD, Emery CL, Lyerly DM. Murray PR, Baron EJ, Jorgensen JH, Pfaller MA, Yolken RH, editors. Clostridium. Manual of clinical microbiology. 2003. 8th ed. Washington, DC: ASM Press;835–856.
2. King BM, Ranck BA, Daugherty FD, Rau CA. Clostridium tertium septicemia. N Engl J Med. 1963. 269:467–469.
3. Miller DL, Brazer S, Murdoch D, Reller LB, Corey GR. Significance of Clostridium tertium bacteremia in neutropenic and nonneutropenic patients: review of 32 cases. Clin Infect Dis. 2001. 32:975–978.

4. Lew JF, Wiedermann BL, Sneed J, Campos J, McCullough D. Aerotolerant Clostridium tertium brain abscess following a lawn dart injury. J Clin Microbiol. 1990. 28:2127–2129.

5. Leegaard TM, Sandven P, Gaustad P. Clostridium tertium: 3 case reports. Scand J Infect Dis. 2005. 37:230–232.

6. Gosbell IB, Johnson CG, Newton PJ, Jelfs J. Clostridium tertium bacteremia: 2 cases and review. Pathology. 1996. 28:70–73.
7. Steyaert S, Peleman R, Vaneechoutte M, De Baere T, Claeys G, Verschraegen G. Septicemia in neutropenic patients infected with Clostridium tertium resistant to cefepime and other expanded-spectrum cephalosporins. J Clin Microbiol. 1999. 37:3778–3779.

8. Thaler M, Gill V, Pizzo PA. Emergence of Clostridium tertium as a pathogen in neutropenic patients. Am J Med. 1986. 81:596–600.
9. Coleman N, Speirs G, Khan J, Broadbent V, Wight DG, Warren RE. Neutropenic enterocolitis associated with Clostridium tertium. J Clin Pathol. 1993. 46:180–183.
10. Valtonen M, Sivonen A, Elonen E. A cluster of seven cases of Clostridium tertium septicemia in neutropenic patients. Eur J Clin Microbiol Infect Dis. 1990. 9:40–42.

11. Speirs G, Warren RE, Rampling A. Clostridium tertium septicemia in patients with neutropenia. J Infect Dis. 1988. 158:1336–1340.
12. Brown EA, Talbot GH, Provencher M, Cassileth P. Anaerobic bacteremia in patients with acute leukemia. Infect Control Hosp Epidemiol. 1989. 10:65–69.

13. Tappe D, Dirks J, Müller R, Brederlau J, Abele-Horn M, Suerbaum S, Kurzai O. Fatal Clostridium tertium septicemia in a nonneutropenic patient. J Infect. 2005. 50:76–80.
14. Vanderhofstadt M, André M, Lonchay C, Levecque P, Holemans X, Canon JL, D'Hondt L. Clostridium tertium bactermia: contamination or true pathogen? A report two cases and a review of the literature. Int J Infect Dis. 2010. 14:Suppl 3. e335–e337.
15. Goldstein EJ, Citron DM, Merriam CV, Warren YA, Tyrrell KL, Fernandez HT. Comparative in vitro susceptibilities of 396 unusual anaerobic strains to tigecycline and eight other antimicrobial agents. Antimicrob Agents Chemother. 2006. 50:3507–3513.

16. Fomin P, Koalov S, Cooper A, Babinchak T, Dartois N, De Vane N, Castaing N, Tellado J. 301 And 306 Study Groups. The efficacy and safety of tigecycline for the treatment of complicated intra-abdominal infections - the European experience. J Chemother. 2008. 20:Suppl 1. 12–19.

17. Babinchak T, Ellis-Grosse E, Dartois N, Rose GM, Loh E. Tigecycline 301 Study Group. Tigecycline 306 Study Group. The efficacy and safety of tigecycline for the treatment of complicated intra-abdominal infections: analysis of pooled clinical trial data. Clin Infect Dis. 2005. 41:Suppl 5. S354–S367.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download