Abstract
Influenza-associated encephalopathy is rare in adults and the role of influenza virus in the pathogenesis of influenza-associated encephalopathy is unclear. We report a case of an adult patient who presented with typical clinical manifestations and magnetic resonance imaging findings of herpes simplex virus (HSV) encephalitis confirmed by positive PCR test in cerebrospinal fluid (CSF), and who was also PCR-positive PCR for H1N1 A/influenza in CSF and a nasopharyngeal swab. The results strongly suggest co-infection of the central nervous system. Given the significant implications for therapeutic interventions and infection control, A/H1N1 influenza should be considered one of the possible etiologies of viral encephalitis when patients present with an influenza-like illness during an influenza epidemic, even in those with typical manifestation of HSV encephalitis.
Although neurological complications of seasonal influenza virus infection have been well described, mostly in children and young adults, such complications are rare in adults infected by influenza virus [1, 2]. Influenza-associated encephalopathy has frequently been reported as a complication of influenza in young children. However, the role of influenza virus in the pathogenesis of influenza-associated encephalopathy has yet to be clarified. Herein, we report on an adult patient who presented with typical clinical manifestations and magnetic resonance imaging (MRI) findings of Herpes simplex virus (HSV) encephalitis confirmed by the positive polymerase chain reaction (PCR) test for HSV in cerebrospinal fluid (CSF), and who also had a positive PCR test for A/H1N1 influenza in CSF and a nasopharyngeal swab. The results strongly suggest that the central nervous system (CNS) of this patient was infected with both viruses.
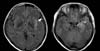
A 43-year-old female presented to our emergency department (ED) with fever and confusion. Three days before presentation to the ED, the patient's daughter was diagnosed with A/H1N1 influenza and the patient also developed fever and flu-like symptoms. The patient took oseltamivir (75 mg every 12 h) and anti-pyretics for 2 days without any improvement. One day before the visit to the ED, the patient developed confusion. The patient had been previously healthy, except for breast cancer, which had completely resolved after a mastectomy and adjuvant radiotherapy 6 years previous. On arrival at the ED, the patient's vital signs were blood pressure, 132/75 mmHg; respiratory rate, 18 breaths per min; and temperature, 37.3℃. The patient was disoriented and not able to recognize family members. Conduction aphasia and neck stiffness were noticed in a language test and neurological examination. The white blood cell (WBC) count was 10,530/µL with neutrophilic dominance (82%). The results of liver and renal function tests were within the normal range. A CSF analysis showed an increased WBC count (40/µL) with dominant lymphocytes (71%) and red blood cell count of 8/µL, normal protein level (38 mg/dL), and a normal CSF:serum glucose ratio (108:150). Magnetic resonance imaging (MRI) showed T2 high-signal intensity in left temporal lobe and insula associated with sulcal hyperintensity (Fig. 1). The dose of oseltamivir was increased (150 mg every 12 h) and intravenous acyclovir (10 mg/kg IV every 8 h) was administered empirically.
On hospital day 2, the patient's confused mentality was aggravated and a diffuse cerebral dysfunction without epileptic discharges was observed in an electroencephalogram. A/H1N1 influenza virus was detected from a nasopharyngeal swab (Cp, 30.59) by real-time PCR using a LightCycler 2.0® (Roche Diagnostics, Mannheim, Germany). Interestingly, PCR testing of CSF for A/H1N1 influenza (Cp, 39.65) and HSV PCR type 1 were both positive. High dose oseltamivir and acyclovir were continued and the patient's aphasia and confusion gradually improved. On hospital day 5, the patient's confused mentality improved significantly and the patient was able to recognize family members. Five days after the administration of antiviral agents, A/H1N1 influenza virus PCR testing of CSF and a nasopharyngeal swab had converted to negative, even though PCR for HSV remained positive. IgG for HSV type I in serum was positive. IgM was negative. Oseltamivir was administered for 7 days and acyclovir was continued for 14 days. Although the patient's initial symptoms had completely resolved and daily activities had resumed, the patient continued to complain of memory disturbance and feeling somewhat slow.
In our report, the positive CSF detection of A/H1N1 influenza virus in this patient presenting as typical HSV encephalitis, and the confirmation of the latter in CSF and MRI suggest CNS involvement with both viruses. It is noted that the patient had PCR results positive for A/H1N1 influenza in both a nasopharyngeal swab sample and CSF specimen, suggesting that the neurological manifestations were more likely to be related to direct CNS invasion of the virus as well as inflammatory responses with cytokine release. The results indicated that viral encephalitis was attributable to a dual infection with novel influenza A virus (H1N1) and HSV. In other words, pharyngitis may be caused by the influenza virus and, simultaneously, encephalitis may be caused by the direct invasion of influenza virus and the reactivation of latent infection with HSV. Although encephalitis in our patient was likely due to the reactivation of HSV, it is still unknown whether latent infection with HSV was reactivated by influenza virus infection, causing encephalitis or not. Influenza-associated encephalopathy attributable to a dual infection with influenza virus and human herpesvirus (HHV) 6 and 7 has also been reported in young children [3]. Among young children with encephalopathy and influenza A virus infection, HHV-6 and/or -7 were demonstrated in CSF specimens by means of PCR, even though the results of PCR testing of CSF specimens for influenza virus were negative [3]. Co-infection with influenza A virus and Epstein-Barr virus infection was also reported in viral encephalitis [4].
Despite neurological complications of seasonal influenza virus infection in young children, such complications are rare in adults infected by influenza virus [1-3, 5]. During the 2009 A/H1N1 pandemic, adult cases with influenza associated acute encephalopathy/encephalitis (IAE) were reported in several countries including South Korea [6-11]. The clinical symptoms of IAE are diverse, and range from typical flu-like symptoms to CNS dysfunction including seizure, altered consciousness, and decreased cognitive processing. The imaging study findings of IAE also had diverse presentations from normal to acute necrotizing encephalitis related to brain lesions of the thalamus, cerebral white matter, brainstem, cerebellum, and parenchyma. The MRI findings in our case showed T2 high-signal intensity in the left temporal lobe and insula, which is a typical manifestation of HSV encephalitis. It could be attributable to main inflammatory mechanisms by HSV in our case with an uncertain role of influenza virus. Since most of IAE cases, however, have been reported to have normal brain image findings despite severe CNS dysfunction, it is hard to conclude that influenza virus had less contribution to the development of meningoencephalitis than HSV in our case by MRI alone.
Our case patient responded well to oseltamivir, but the emergence of oseltamivir-resistance in Korea is challenging to physicians who are struggling with treatment for influenza encephalitis [12]. Apart from antiviral therapy with oseltamivir or amanatdine, mild hypothermia therapy and anticytokine agents may be effective for severe IAE cases [13].
To our knowledge, this is the first reported case of an adult presenting with encephalitis caused by co-infection of H1N1 influenza and HSV. Given the significant implications for therapeutic interventions and infection control, A/H1N1 influenza should be considered one of the possible etiologies of viral encephalitis when patients present with influenza-like illness during an influenza epidemic, even in those with typical manifestation of HSV encephalitis.
Figures and Tables
References
1. Centers for Disease Control and Prevention (CDC). Neurologic complications associated with novel influenza A (H1N1) virus infection in children - Dallas, Texas, May 2009. MMWR Morb Mortal Wkly Rep. 2009. 58:773–778.
2. Morishima T, Togashi T, Yokota S, Okuno Y, Miyazaki C, Tashiro M, Okabe N. Collaborative Study Group on Influenza-Associated Encephalopathy in Japan. Encephalitis and encephalopathy associated with an influenza epidemic in Japan. Clin Infect Dis. 2002. 35:512–517.

3. Sugaya N, Yoshikawa T, Miura M, Ishizuka T, Kawakami C, Asano Y. Influenza encephalopathy associated with infection with human herpesvirus 6 and/or human herpesvirus 7. Clin Infect Dis. 2002. 34:461–466.

4. Studahl M, Bergström T, Hagberg L. Acute viral encephalitis in adults--a prospective study. Scand J Infect Dis. 1998. 30:215–220.

5. Kasai T, Togashi T, Morishima T. Encephalopathy associated with influenza epidemics. Lancet. 2000. 355:1558–1559.

6. Chen YC, Lo CP, Chang TP. Novel influenza A (H1N1)-associated encephalopathy/encephalitis with severe neurological sequelae and unique image features--a case report. J Neurol Sci. 2010. 298:110–113.

7. Choi SY, Jang SH, Kim JO, Ihm CH, Lee MS, Yoon SJ. Novel swine-origin influenza A (H1N1) viral encephalitis. Yonsei Med J. 2010. 51:291–292.

9. Gonzalez BE, Brust DG. Novel influenza A (H1N1) presenting as an acute febrile encephalopathy in a mother and daughter. Clin Infect Dis. 2009. 49:1966–1967.

10. Kitcharoen S, Pattapongsin M, Sawanyawisuth K, Angela V, Tiamkao S. Neurologic manifestations of pandemic (H1N1) 2009 virus infection. Emerg Infect Dis. 2010. 16:569–570.

11. Lee N, Wong CK, Chan PK, Lindegardh N, White NJ, Hayden FG, Wong EH, Wong KS, Cockram CS, Sung JJ, Hui DS. Acute encephalopathy associated with influenza A infection in adults. Emerg Infect Dis. 2010. 16:139–142.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download