Abstract
The human metapneumovirus virus (hMPV) is a recently described human respiratory pathogen. The virus has usually been associated with upper and lower respiratory tract infections in children. Since most of the available data on the clinical manifestations come from observational studies on children, relatively little is known of emerging hMPV infections in the adult population in Korea. A 32-year old female patient, presenting fever, chills, cough and sputum for 3 days progressed to severe pneumonia during the season of pandemic influenza A/H1N1-2009. RT-PCR screening of viral pathogens revealed hMPV. Clinical improvement was achieved a week after illness. This case represents severe hMPV pneumonia developed in an immunocompetent adult.
Human metapneumovirus virus (hMPV) was first isolated in 2001 from 28 young children with symptomatic respiratory infection similar to that of the human respiratory syncytial virus (hRSV) [1]. Genetically, hMPV is related to avian pneumovirus and has been classified in the subfamily Pneumovirinae, genus Metapneumovirus [2]. Recent studies from Canada and England have updated the age-range of symptomatic patients identified by culture and/or by reverse transcription-polymerase chain reaction (RT-PCR), from 2 months to 87 years old [3]. According to a prospective study on hMPV pneumonia during the respiratory virus season in adults, hMPV was the causative organism in 4% of all community acquired pneumonia [4], which indicates the increasing burden of hMPV in the adult population. However, most infection in healthy young adults still results in mild upper respiratory infections or even asymptomatic infections [5]. The presentation of pneumonia due to hMPV in adults has not been well characterized, and the incidence may be underestimated. We report an experience of severe hMPV pneumonia in an immunocompetent adult during the influenza season.
A 32-year-old woman was admitted to our hospital after she suffered from fever, chilling, cough and sputum over the course of 3 days in April 2010. On admission, her body temperature was 38.5℃, blood pressure was 120/80 mmHg, heart rate was 88 beats/min, and respiratory rate was 20 breaths/min. On physical examination, she showed an acute ill looking appearance, and rale was heard bilaterally in the lungs. Laboratory findings were as follows: white blood cell count 5,000/µL with differentials of segmented neutrophils 82%, lymphocytes 14%, monocyte 2%, and eosinophil 1%, hemoglobin 12.7 g/dL, platelet 100,000/µL, and C-reactive protein 4.08 mg/dL. Chest radiography showed patchy increased opacity in right mid to lower lung field (Fig. 1). Chest computed tomography scan showed segmental and lobular consolidation with ground glass attenuation in right lung and left lower lobe, and a greater extent and severity in both lower lobes. In addition, suspicious small lymphadenopathy at both mediastinum was shown (Fig. 2). The findings were suggestive of bacterial or viral pneumonia. Under a presumptive diagnosis of community-acquired pneumonia, ceftriaxone 2 g/day, azithromycin 250 mg bid were administered. On the 4th day of admission, arterial blood gas analysis showed pH 7.39, PaO2 46.8 mmHg, PaCO2 37.7 mmHg, SaO2 82.9 % and (A-a) DO2 was 56 at room air. The PaO2/FiO2 ratio was 222 (<250) and a chest radiograph showed multilobar pneumonia. The patient was advised to go to the Intensive Care Unit (ICU) care, but she and her family did not agree. Her respiratory status progressively worsened and she required O2 inhalation at 6 L/min by reservoir mask. On the 4th day of admission, moxifloxacin 400 mg/day, oseltamivir 300 mg, amantadine 100 mg bid, and ribavirin 100 mg tid were administered under suspicion of atypical pneumonia including the possibility of the influenza A/H1N1-2009, although the peak wave has been waning. The blood and sputum cultures failed to reveal the causative organisms for pneumonia. The sputum Gram stain showed few Gram positive cocci, Gram negative rod, diplococcic and sputum culture showed alpha hemolytic streptococcus, Neisseria. The RT-PCR assay and antigen detection method for pandemic influenza A/H1N1-2009 were both negative. RT-PCR assays of induced sputum were performed for the detection of other viral pathogens, which resulted positive for hMPV. The patient was a nurse in a hospital, but in her place of work there was no one with respiratory viral infection at that time. On the 6th day of admission, the fever subsided, and neutropenia and thrombocytopenia were almost recovered along with the improvement of clinical symptoms, the chest radiograph was also markedly improved (Fig. 3). On the 14th day, she was discharged without complications.
The hMPV is one of the newer viral respiratory pathogens, first identified in 2001 in the Netherlands [1]. The epidemiology and clinical characteristics of hMPV have yet to be discovered. This virus has been associated with both upper and lower respiratory tract infections, and is responsible for significant proportions of respiratory illness in children and adults [6-8]. Most of the available data on the clinical manifestations of hMPV infection are from studies of viral upper respiratory tract infections in children [5]. The incidence of symptomatic infection in the adult population is relatively low and is typically less than 5% in most studies [4, 9, 10]. The infection is usually associated with high rates of cough (100%), nasal congestion (85%), dyspnea (69%), and wheezing (62%). Several studies suggest that hMPV infection is an important cause of respiratory infections in young healthy adults [4, 5]. The outcome of community acquired pneumonia due to hMPV in adults was favorable. In a prospective cohort study, a total of 300 patients were enrolled and 193 had evaluable nasopharyngeal samples. Eight (4%) of 193 patients had hMPV RNA detected. No patients died or required admission to an intensive care unit [4]. Adults at highest risk of serious sequelae because of hMPV included the elderly, adults with underlying pulmonary disease, and those who were immunocompromised [5]. Older adults experienced significantly more dyspnea and wheezing, compared with young people [11]. In 2009, the first case of severe hMPV pneumonia in an immunocompetent adult after travel to China was reported in Korea [12]. We report a case of a young healthy woman who showed severe hMPV pneumonia in Korea during the pandemic influenza season without recent history of international travel. The Infectious Disease Society of America (IDSA) and the American Thoracic Society (ATS) guidelines issued in 2007 defines severe community acquired pneumonia. The guideline recommended admission to the ICU if the patient suffers septic shock or requires mechanical ventilation. ICU admission was also warranted if patients had 3 of the minor criteria for severe CAP, including a respiratory rate of 30/min or more, PaO2/FIO2 ≤250, multilobar infiltrates, confusion, uremia, neutropenia, thrombocytopenia, and hypothermia. In our case, the patient fulfilled criteria for severe pneumonia and ICU admission, although the patient initially refused to be treated in the ICU. The patient's clinical manifestations were indistinguishable from influenza or RSV infection. Johnstone et al. reported that hMPV pneumonia seemed to be distinguishable from influenza because of the absence of fever, myalgia, and gastrointestinal symptoms [4]. In our case, the patient suffered from fever, chilling, cough, sputum and had no leukocytosis, so we were suspicious of respiratory viral infection, especially Influenza. A prospective study investigated the clinical and epidemiological differences between hMPV and RSV in Korean children from December 2003 to April 2008 [11]. There were significant differences in age distribution between the hMPV and RSV patients. The mean age of patients with hMPV was greater than the age of RSV patients. RSV patients had significantly higher eosinophil counts than hMPV patients. However, there was no significant difference in neutrophil counts. Analysis of the seasonality of these viral infections showed that there is a general trend towards both RSV and hMPV occurring mainly in the fall and winter following in the spring, although both viruses are perennially identifiable in Korea [11]. The hMPV tends to peak at spring, whereas RSV peaks at fall or at early winter in Korean children [13]. It has previously been shown that hMPV is associated with higher mortality in immunocompromised adults, so rapid diagnosis might be a challenging clinical issue. Standardized viral antigens are needed for serologic surveys. An enzyme-linked immunosorbent assay (ELISA) was developed, which has been tested in adults and children [14]. Up to now, the laboratory diagnosis of hMPV infections is mainly based on detection of nucleic acid by PCR. Rapid diagnosis of hMPV by direct immunofluorescence (DIF) staining of cells from nasopharyngeal secretions offers advantages for some laboratories. Recent studies have indicated that the sensitivity of DIF using hMPV-specific monoclonal antibodies is in the same range of RT-PCR [13, 15]. Althought hMPV is not a common pathogen in the community, it should be considered for differential diagnosis of adult atypical pneumonia. Treatment is supportive and varies with the clinical manifestations. Although there is no pharmaceutical agent approved for treatment of hMPV pneumonia, there are a few cases that have demonstrated clinical resolution of symptoms using intravenous ribavirin and immunoglobulin. Kamble RT et al. reported the first successful treatment of hMPV pneumonia in a hematopoietic stem cell transplantation recipient with intravenous ribavirin and immunoglobulin [16]. In addition, Bonney et al. reported a case of a child on chemotherapy for acute lymphoblastic leukemia with proven hMPV pneumonia, using ribavirin and immunoglobulin [17]. Also, there are data from in vitro studies that give theoretical support to the use of ribavirin. In Hamelin et al. ribavirin significantly decreased both hMPV replication in lung and pulmonary inflammation [18]. This in vitro study and the few case reports may make this therapy worth considering. Further research on early diagnosis will improve treatment outcomes, while reducing the use of unnecessary antibiotics. It may also aid in limiting transmission of hMPV, particularly to immunocompromised hosts. A retrospective study of hMPV infections in different populations are necessary to determine the relative risks, as well as the clinical spectrum of hMPV associated diseases.
Figures and Tables
Figure 1
Chest radiograph of patient at admission and 4th day of admission. (A) The initial chest radiograph shows increased opacity in right mid to lower lung field. (B) After 4 days, the chest radiograph shows diffuse increased opacity in both lungs.
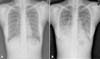
Figure 2
Chest computed tomography scan of the patient showed consolidations with ground glass attenuation in both lobes.

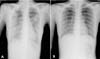
Figure 3
The radiologic findings of the patient with hMPV pneumonia were markedly improved during admission. (A) On the 7th hospital day, the chest radiograph showed gradually developing diffuse increased opacity in both lung. (B) On the 11th hospital day, there is no active lesion in both lung fields.
References
1. van den Hoogen BG, de Jong JC, Groen J, Kuiken T, de Groot R, Fouchier RA, Osterhaus AD. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001. 7:719–724.

2. van den Hoogen BG, Bestebroer TM, Osterhaus AD, Fouchier RA. Analysis of the genomic sequence of a human metapneumovirus. Virology. 2002. 295:119–132.

3. Peret TC, Boivin G, Li Y, Couillard M, Humphrey C, Osterhaus AD, Erdman DD, Anderson LJ. Characterization of human metapneumoviruses isolated from patients in North America. J Infect Dis. 2002. 185:1660–1663.

4. Johnstone J, Majumdar SR, Fox JD, Marrie TJ. Human metapneumovirus pneumonia in adults: results of a prospective study. Clin Infect Dis. 2008. 46:571–574.

5. Falsey AR. Human metapneumovirus infection in adults. Pediatr Infect Dis J. 2008. 27:10 Suppl. S80–S83.

6. Falsey AR, Erdman D, Anderson LJ, Walsh EE. Human metapneumovirus infections in young and elderly adults. J Infect Dis. 2003. 187:785–790.

7. Fouchier RA, Rimmelzwaan GF, Kuiken T, Osterhaus AD. Newer respiratory virus infections: human metapneumovirus, avian influenza virus, and human coronaviruses. Curr Opin Infect Dis. 2005. 18:141–146.

8. Mackay IM, Jacob KC, Woolhouse D, Waller K, Syrmis MW, Whiley DM, Siebert DJ, Nissen M, Sloots TP. Molecular assays for detection of human metapneumovirus. J Clin Microbiol. 2003. 41:100–105.

10. van den Hoogen BG, Osterhaus AD, Fouchier RA. Clinical impact and diagnosis of human metapneumovirus infection. Pediatr Infect Dis J. 2004. 23:1 Suppl. S25–S32.

11. Kim CK, Choi J, Callaway Z, Kim HB, Chung JY, Koh YY, Shin BM. Clinical and epidemiological comparison of human metapneumovirus and respiratory syncytial virus in Seoul, Korea, 2003-2008. J Korean Med Sci. 2010. 25:342–347.

12. Lim HJ, Lee JW, Park YS, Kim NH, Kim M, Yim JJ, Yang SC, Yoo CG, Kim YW, Han SK, Shim YS, Lee SM. A case of severe human metapneumovirus pneumonia requiring mechanical ventilation in an immunocompetent adult. Tuberc Respir Dis. 2009. 67:135–139.

13. Kim YK, Kim JW, Wee YS, Yoo EG, Han MY. Clinical features of human metapneumovirus and respiratory syncytial virus infection in hospitalized children. Pediatr Allergy Respir Dis. 2009. 19:12–19.
14. Hamelin ME, Boivin G. Development and validation of an enzyme-linked immunosorbent assay for human metapneumovirus serology based on a recombinant viral protein. Clin Diagn Lab Immunol. 2005. 12:249–253.

15. Manoha C, Bour JB, Pitoiset C, Darniot M, Aho S, Pothier P. Rapid and sensitive detection of metapneumovirus in clinical specimens by indirect fluorescence assay using a monoclonal antibody. J Med Virol. 2008. 80:154–158.

16. Kamble RT, Bollard C, Demmler G, LaSala PR, Carrum G. Human metapneumovirus infection in a hematopoietic transplant recipient. Bone Marrow Transplant. 2007. 40:699–700.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download