Abstract
Typhoid fever is a class I legally designated communicable disease in Korea; and if remains as an important public health problem in many developing countries. It takes at least 3-5 days to detect and identify Salmonella Typhi (S. Typhi) by classical diagnostic method. For this reason, multiplex PCR (mPCR) was evaluated in detecting and identifying S. Typhi. In this study, forty-three bacterial strains, which consisted of 42 Salmonella enterica serovars and one Citrobacter freundii. were used to evaluate the promptness of mPCR in detecting and identifying S. Typhi. mPCR was performed with four genes which were known for representing Salmonella spp and/or S. Typhi: invA, fliC-d, viaB and prt. invA and prt gene was amplified in all strains and viaB gene was in only S. Typhi. fliC-d gene was amplified in three serovars: S. Typhi, S. Schwarzengrund and S. Livingstone. After specificity test, mPCR was modified as triplex PCR with three genes (invA, fliC-d, and viaB) and the sensitivity test was performed against S. Typhi-inoculated stool samples. mPCR was able to detect S. Typhi cell suspension of 1×105 cfu/mL. We found that modified multiplex PCR was useful to detect S. Typhi from stool samples within 24h whereas it takes 3-5days to detect by classic diagnosis method.
Figures and Tables
Figure 1
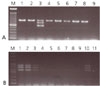
The specificity and sensitivity of modified multiplex PCR to detect S. Typhi. (A) Specificity of multiplex PCR. invA gene was amplified all Salmonella serovars but viaB and fliC-d was only S. Typhi by modified multiplex PCR. All Salmonella serovar was inoculated into human feces, concentration of 108 cfu/mL. M. 100 bp DNA ladder; Lane 1. S. Enteritidis; Lane 2. S. Typhimurium; Lane 3. S. Typhi; Lane 4, S. Braenderup; Lane 5, S. London; Lane 6. S. ParatyphiA; Lane 7, S. Infantis; Lane 8, S. Montevideo; Lane 9, Negative control (distiled water). (B) Sensitivity of multiplex PCR. Modified multiplex PCR was able to detect 105 cfu/mL of S. Typhi, which contained human feces. M, 100bp DNA ladder; Lane 1, 107 cfu/mL; Lane 2, 106 cfu/mL; Lane 3, 105 cfu/mL; Lane 4, 104 cfu/mL; Lane 5, 103 cfu/mL; Lane 6, 102 cfu/mL; Lane 7, 101 cfu/mL; Lane 8, 100 cfu/mL; Lane 9, 0 cfu/mL; Lane 10, Positive control; Lane 11, Negative control (distiled water).
References
1. Infectious Disease Web Statics, Surveillance data of Typhoid fever in Korea. KCDC. Accessed 10 February 2010. Available at: http://stat.cdc.go.kr.
2. Lim BK, Thong KL. Application of PCR-based serogrouping of selected Salmonella serotypes in Malaysia. J Infect Dev Ctries. 2009. 3:420–428.

3. Hatta M, Smits HL. Detection of Salmonella typhi by nested polymerase chain reaction in blood, urine, and stool samples. Am J Trop Med Hyg. 2007. 76:139–143.

4. Prakash P, Mishra OP, Singh AK, Gulati AK, Nath G. Evaluation of nested PCR in diagnosis of typhoid fever. J Clin Microbiol. 2005. 43:431–432.

5. Lin CL, Chiu CH, Chu C, Huang YC, Lin TY, Ou JT. A multiplex polymerase chain reaction method for rapid identification of Citrobacter freundii and Salmonella species, including Salmonella Typhi. J Microbiol Immunol Infect. 2007. 40:222–226.
6. Levy H, Diallo S, Tennant SM, Livio S, Sow SO, Tapia M, Fields PI, Mikoleit M, Tamboura B, Kotloff KL, Lagos R, Nataro JP, Galen JE, Levine MM. PCR method to identify Salmonella enterica serovars Typhi, Paratyphi A, and Paratyphi B among Salmonella isolates from the blood of patients with clinical enteric fever. J Clin Microbiol. 2008. 46:1861–1866.

7. Park SH, Kim HJ, Cho WH, Kim JH, Oh MH, Kim SH, Lee BK, Ricke SC, Kim HY. Identification of Salmonella enterica subspecies I, Salmonella enterica serovars Typhimurium, Enteritidis and Typhi using multiplex PCR. FEMS Microbiol Lett. 2009. 301:137–146.

8. Octavia S, Lan R. Single-nucleotide-polymorphism typing and genetic relationships of Salmonella enterica serovar Typhi isolates. J Clin Microbiol. 2007. 45:3795–3801.

9. Kumar S, Balakrishna K, Batra HV. Detection of Salmonella enterica serovar Typhi (S. Typhi) by selective amplification of invA, viaB, fliC-d and prt genes by polymerase chain reaction in mutiplex format. Lett Appl Microbiol. 2006. 42:149–154.

10. Sambrook J, Russell DW. Molecular cloning: a laboratory manual. 2001. 3rd ed. New York: Cold Spring Harbor Laboratory Press;1.43–1.50.
11. Teh CS, Chua KH, Puthucheary SD, Thong KL. Further evaluation of a multiplex PCR for differentiation of Salmonella paratyphi A from other salmonellae. Jpn J Infect Dis. 2008. 61:313–314.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download