Abstract
Progressive multifocal leukoencephalopathy (PML) is a demyelinating disease of the central nervous system due to JC virus. In acquired immunodeficiency syndrome (AIDS) patients, JC virus infects myelin-producing oligodendrocytes causing a non-inflammatory lytic reaction leading to demyelination and brain death. We herein report a case of a 56-years-old AIDS man who developed immune reconstitution inflammatory syndrome and died while undergoing highly active antiretroviral therapy. In this patient, the PML involved the brainstem, causing mental confusion followed by recurrent aspiration, adult respiratory distress syndrome, and eventually to early death.
Neurologic disease is the initial manifestation of acquired immunodeficiency syndrome (AIDS) in 10-20% of patients with human immunodeficiency virus (HIV) infection (1). Progressive multifocal leukoencephalopathy (PML) is a demyelinating disease of the central nervous system (CNS) due to human papovavirus, called JC virus (1-3). It is the reactivation of latent JC virus infection in the setting of cellular immuno-deficiencies (2). Before the AIDS pandemic, PML was a rare disorder and this was most often due to chronic lymphatic leukemia, lymphoma, or other neoplasms. As a consequence of the AIDS pandemic, the incidence of PML has increased dramatically and occurs mostly (in about 85%) in HIV-infected patients (1, 3). In AIDS patients, JC virus infects myelin-producing oligodendrocytes in the white matter of the CNS causing a non-inflammatory lytic reaction leading to demyelination, necrosis, and cell death of the brain (1, 3). The most common places where PML occurs are in the frontal, parietal, and occipital lobes (3).
Highly active antiretroviral therapy (HAART) is the only effective therapy for PML in HIV infected patients (2, 4). Since the advent of HAART, prognosis for PML has much improved. However, a significant number of patients are unresponsive to antiretroviral therapy and some worsen as a consequence of the development of immune reconstitution inflammatory syndrome (IRIS) (2, 4).
We herein report a case of a 56-year-old AIDS man who presented with PML, developed IRIS, and resulted in death.
A 56-year-old man with no significant past medical history presented with 3 week history of dysarthria and right sided upper motor weakness. On arrival, the patient was afebrile and vital signs were all normal: blood pressure, 130/80 mmHg; heart rate, 89/min; respiratory rate, 21/min; body temperature, 36.9℃. On neurologic examination, there was dysarthria, right upper extremity weakness (grade IV), and right side ataxia. The results of laboratory studies were as follows: hemoglobin, 14.4 g/dL; hematocrit, 42.1%; white blood cell count, 4,480/µL (granulocyte, 46%; monocyte, 5%; lymphocyte, 44%); platelet, 129,000/µL. Magnetic resonance imaging (MRI) taken on hospital day 1 showed focal patchy high signal intensity lesion at the right anterior cerebellar hemisphere in T2-weighted image, but focal patchy low signal intensity lesion at the right anterior cerebellar hemisphere in T1-weighted image (Fig. 1). MR spectroscopy showed slightly increased choline peak (3.2 ppm), lactate peak (1.35 ppm), and decreased N-acetylaspartate (NAA) peak (2.0 ppm), suggesting active demyelinating disease or etiologies (Fig. 2). A cerebral spinal fluid (CSF) findings showed the following results: glucose, 61 mg/dL; protein, 44.7 mg/dL; white blood cell, 0/µL. The result of gram stain, india ink stain, and acid fast bacilli stain was all negative. A CSF polymerase chain reaction (PCR) for JC virus DNA was positive indicating the presence of PML. PCR was done at the department of laboratory medicine, Hangang Sacred Heart Hospital, Hallym University; a total of 200 µL of CSF was incubated for 3 hours at 55℃ with 200 µL of 5% SDS and 10 µL of proteinase K (20 mg/mL). The DNA was extracted once with an equal volume of phenol/chloroform/isoamyl alcohol mixture and once with chloroform/isoamyl alcohol (Bioneer, Korea). The precipitated DNA was dissolved with 10 µL of sterile double distilled water. Primers and PCR amplification tests for JC virus were performed as described by Fedele et al. (5). The outer primers were designed to amplify a conserved DNA region of the large T antigen gene of polyomavirus. For the amplification of the T antigen gene, primers PM1+ (5'-TCY TCT GGN NTA AAR TCATGCTCC-3') and PM1-(5'-AAW TAG RTK CCA ACC TAT GGA AC-3') were used. The inner primers specific for JC virus were PM2- (5'-GGT AGA ATA CCC YAA RGACTTTCC-3') and JV+ (ATA TTA TGA CCMCCAAAACCATG-3'). Nested PCR products were resolved on a 4% agarose gel by electrophoresis. The presence of the 189 bp band on agarose was considered positive for JC virus.
The patient's HIV-1 antibody test was positive with a CD4+ T-lymphocyte count of 189/µL and an HIV-1 RNA level of 1,183,752 copies/mL. Herpes simplex virus, cytomegalovirus, toxoplasma, varicella, Grocott's methenamine silver nitrate stain of sputum for Pneumocystis jiroveci, Treponema pallidum hemagglutination assay, and venereal disease research laboratory (VDRL) were checked and all antibodies were negatives. Antiretrovial therapy including lopinavir/ritonavir and lamivudine/zidovudine were started. MRI was taken on hospital day 22 showed that, in the T2-weighted image interval, the extent of previously seen patchy high signal intensity lesion at the right anterior cerebellar hemisphere has increased. The extent of previously seen patchy low signal intensity lesion at the right anterior cerebellar hemisphere was also been shown to have increased in the T1-weighted image interval. Both MRI images indicated interval aggravation (Fig. 3). After 4 weeks of HAART, CD4+ T-lymphocyte count slightly increased (304/µL), but the symptoms worsened, manifested by diplopia, ataxia, and finally leading to gait disturbance. Six weeks after the commencement of HAART, general fever, disturbance of consciousness, and recurrent aspiration developed which resulted in pneumonia. The patient eventually passed away three days later after developing adult respiratory distress syndrome (ARDS). The autopsy could not be undertaken because of family refusal.
PML was first described in 1958 in three patients with underlying lymphoproliferative disorders (6, 7). This unique neurologic disorder was characterized chiefly by a triad of unique histopathologic features that included demyelination, abnormal oligodendroglial nuclei, and giant astrocytes (6, 8, 9).
PML results in a variety of neurologic symptoms including visual impairment or blindness, and motor dysfunction or weakness (10). In our patient, dysarthria, visual impairment, motor weakness, ataxia, and dizziness were observed.
The diagnosis of PML is based on characteristic progression of central symptoms, demonstration of JC virus in the CSF by PCR, and typical radiological findings, although the biopsy remains the gold standard for establishing the diagnosis (11). In our case, the patient was diagnosed with PML based on neurologic symptoms, JC virus in the CSF by PCR, typical radiological findings, but not brain biopsy.
Especially, neuro-imaging is very helpful in establishing the diagnosis. For example, MRI of the brain in PML reveals hyperintense signal abnormalities on T2 weighted or hypointense signal abnormalities on T1 weighted image (6, 8, 12, 13). Also, faint peripheral enhancement with gadolinium may rarely be observed in about 5-10% (6, 13). Mass effect is unanticipated, but may occur as may florid contrast enhancement in the setting of the IRIS (13). PML predominantly involves the cerebral hemispheres with one-third of patients having concomitant cerebellar lesions (1, 3). A small minority of patients have lesions confined to the cerebellum (1). In our case, the lesions of PML on MRI were located in the cerebellum including bilateral pons, which was unusual.
Recent progress in newer imaging techniques, like MR spectroscopy, is replacing the invasive methods for diagnosing PML (6, 12). Magnetic resonance spectroscopy in PML reveals a decrease in NAA and creatine, and an increase in choline products, myoinositol, and lactate (6). These findings reflect neuronal loss and breakdown of cell membrane and myelin, consequent to PML (6). In our case, MRI brain spectroscopy revealed slightly increased choline peak (3.2 ppm), lactate peak (1.35 ppm), with decreased NAA peak (2.0 ppm) which were compatible with PML.
PCR analysis of CSF for JC virus is the best non-invasive test to confirm PML, because several studies have confirmed that CSF PCR testing for JC virus in PML has high sensitivity and specificity (12). In our case, a CSF PCR for JC virus DNA was positive.
Prior to active HAART, there was no effective therapy for PML. Recently, several studies have proved that patients with PML have a significant survival benefit from HAART. However, about 20% of patients were unresponsive to anti-retroviral therapy and some of them worsen because of the development of IRIS (2, 4, 9). The robust immune response by IRIS develops against the previously encountered infectious agent to which the patient's immune system, prior to the onset of potent HAART, was unable to mount an effective response secondary to immunosuppression (1). In the case report by D'amico et al., increased in the size of the lesions indicate that the inflammatory response associated with PML-IRIS occurs in areas of the brain that, prior to potent HAART, may have harbored JC virus in a latent or quiescent state (1). The diagnosis of IRIS in patients receiving HAART is considered with rising CD4 cell count and falling HIV-1 RNA level (2) as have occurred in our patient. Several viral and host factors may play a role in the clinical outcome of AIDS patients with PML-IRIS. One is the baseline CD4+ T-lymphocyte cell count at the time of HAART initiation. Patients with CD4+T-lymphocyte >300 cells/mm3 show improved survival. Another is the cellular immune response generated by the host to JC virus infection. PML survivor showed detectable cytotoxic T-lymphocyte response. Infecting viral strain and alterations of other factors in the regulatory region of the viral genome VP1 gene are also associated with PML (14-16). The brainstem was involved by PML-IRIS in our patient. As has been reported in a previous study, patients with brainstem involvement were significantly more likely to die of any cause (17).
Since there still are differing opinions about adding cidofovir or corticosteroids to the treatment of PML (3, 17-19), we did not prescribe cidofovir or corticosteroids to our patient.
Unfortunately, evidence based guidelines for prevention or management of PML-IRIS are still lacking. New agent and treatment strategies are needed for the treatment of PML-IRIS.
In summary, a 56-year-old man with 3 weeks' history of dysarthria and right upper motor weakness was diagnosed with AIDS and antiretroviral therapy was started immediately. Although CD4+ T-lymphocyte count increased after initiating HAART, his symptoms worsened and the patient eventually passed away after being treated for six weeks.
Figures and Tables
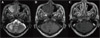 | Figure 1Initial brain MRI. Focal patchy high and low signal intensity lesion are seen at right anterior cerebellar hemisphere on T2-weighted image (A) and T1-weighted image (B). (C) Postcontrast-T1-signal intensity. There is no demonstrable enhancement at the right anterior cerebellar hemisphere. |
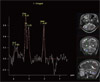 | Figure 2MR spectroscopy. MR spectroscopy show slightly increased choline peak (3.2 ppm), lactate peak (1.35 ppm), and decreased N-acetylaspartate (NAA) peak (2.0 ppm), suggestive of active demyelinating disease. |
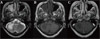 | Figure 3Follow-up brain MRI 3 weeks later. (a) T2-weighted image. Interval increased extent of previously seen patchy high signal intensity lesion is seen at the right anterior cerebellar hemisphere, which shows interval aggravation. (b) T1-weighted image. Interval increased extent of previously seen patchy low signal intensity lesion is seen at the right anterior cerebellar hemisphere, which shows interval aggravation. (c) Postcontrast-T1-signal intensity. There is no demonstrable enhancement at the right anterior cerebellar hemisphere. |
References
1. D'Amico R, Sarkar S, Yusuff J, Azar E, Perlman DC. Immune reconstitution after potent antiretroviral therapy in AIDS patients with progressive multifocal leukoencephalopathy. Scand J Infect Dis. 2006. 39:347–350.
2. Martinez JV, Mazziotti JV, Efron ED, Bonardo P, Jordan R, Sevlever G, Martinez M, Verbanaz SC, Salazar ZS, Pardal MF, Reisin R. Immune reconstitution inflammatory syndrome associated with PML in AIDS: a treatable disorder. Neurology. 2006. 67:1692–1694.

3. Kraemer C, Evers S, Nolting T, Arendt G, Husstedt IW. Cidofovir in combination with HAART and survival in AIDS-associated progressive multifocal leukoencephalopathy. J Neurol. 2008. 255:526–531.

4. Gray F, Chrétien F, Vallat-Decouvelaere AV, Scaravilli F. The changing pattern of HIV neuropathology in the HAART era. J Neuropathol Exp Neurol. 2003. 62:429–440.

5. Fedele CG, Ciardi M, Delia S, Echevarria JM, Tenorio A. Multiplex polymerase chain reaction for the simultaneous detection and typing of polyomavirus JC, BK and SV40 DNA in clinical samples. J Virol Methods. 1999. 82:137–144.

6. Berger JR. Progressive multifocal leukoencephalopathy. Curr Neurol Neurosci Rep. 2007. 7:461–469.

7. Chukwudelunzu FE. Progressive multifocal leukoencephalopathy as an initial manifestation of AIDS. Hosp Physician. 2001. 5:65–70.
8. Lee MH, Chen YZ, Wang LS, Yen PS, Hsu YH. Progressive multifocal leukoencephalopathy in an AIDS patient. J Formos Med Assoc. 2007. 106:Suppl 3. S24–S28.

9. Aksamit AJ. Progressive multifocal leukoencephalopathy. Curr Treat Options Neurol. 2008. 10:178–185.

10. Major EO, Amemiya K, Tornatore CS, Houff SA, Berger JR. Pathogenesis and molecular biology of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clin Microbiol Rev. 1992. 5:49–73.

11. Koralnik IJ. New insights into progressive multifocal leukoencephalopathy. Curr Opin Neurol. 2004. 17:365–370.

12. Raina S, Kaushal SS, Gupta D, Himral P, Sawal N, Sood V, Goyal A. Progressive multifocal leukoencephalopathy--as a presenting manifestation of AIDS. J Assoc Physicians India. 2007. 55:797–801.
13. Berger JR, Houff S. Progressive multifocal leukoencephalopathy: lessons from AIDS and natalizumab. Neurol Res. 2006. 28:299–305.

14. Berger JR, Levy RM, Flomenhoft D, Dobbs M. Predictive factors for prolonged survival in acquired immunodeficiency syndrome-associated progressive multifocal leukoencephalopathy. Ann Neurol. 1998. 44:341–349.

15. Koralnik IJ, Du Pasquier RA, Letvin NL. JC virus-specific cytotoxic T lymphocytes in individuals with progressive multifocal leukoencephalopathy. J Virol. 2001. 75:3483–3487.

16. Agostini HT, Ryschkewitsch CF, Stoner GL. Genotype profile of human polyomavirus JC excreted in urine of immunocompetent individuals. J Clin Microbiol. 1996. 34:159–164.

17. De Luca A, Ammassari A, Pezzotti P, Cinque P, Gasnault J, Berenguer J, Di Giambenedetto S, Cingolani A, Taoufik Y, Miralles P, Marra CM, Antinori A. Gesida 9/99, IRINA, ACTG 363 Study Groups. Cidofovir in addition to antiretroviral treatment is not effective for AIDS-associated progressive multifocal leukoencephalopathy: a multicohort analysis. AIDS. 2008. 22:1759–1767.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download