Abstract
Background
Invasive aspergillosis (IA) is associated with significant morbidity and mortality in patients with hematologic malignancies. We investigated the efficacy and safety of voriconazole (VCZ) when used as salvage therapy for IA in Korean adults with hematologic malignancies who had not responded to prior antifungal therapy.
Materials and Methods
We retrospectively reviewed data, collected from January 2007 to October 2008, from patients with proven or probable cases of IA. All were probable IA cases, except for one proven case. All cases were refractory or intolerant to antifungal therapy prior to administration of VCZ. Efficacy and safety were assessed in patients treated with VCZ for more than 3 days and for more than one dose, respectively. A favorable response [complete (CR) or partial (PR)] was defined by significant improvement of all clinical symptoms, signs, and radiologic abnormalities.
Results
Fifty patients who met the inclusion criteria were enrolled. There were 27 male and 23 female patients with mean age of 44.4 years (range, 15-65 years). Underlying diseases were acute leukemia (35 cases), chronic myelogenous leukemia (4 cases), myelodysplastic syndrome (3 cases), lymphoma (3 cases) and other hematologic diseases (5 cases). Twenty-two patients had received chemotherapy and 13 patients had undergone hematopoietic stem cell transplantation. The lung was the main infection site (94%) followed by the sinus (6%). Amphotericin B deoxycholate alone was the most frequent previous antifungal therapy. The mean duration of antifungal therapy prior to VCZ therapy was 13.9±8.8 days (2-44 days). The median duration of VCZ therapy was 19 days (interquartile range, 49 days). Sixteen patients (32.0%) showed favorable responses (CR:PR=8:8) at the end of VCZ therapy. The numbers of patients with stable disease, progression and death were, 6 (12%), 6 (12%) and 22 (44%) respectively. Most of those with unfavorable responses had relapsed underlying malignancies or refractory graft versus host diseases. Twelve patients developed drug-related adverse events but only one patient stopped VCZ treatment prematurely.
References
1. Maschmeyer G, Haas A, Cornely OA. Invasive aspergillosis: epidemiology, diagnosis and management in immunocompromised patients. Drugs. 2007; 67:1567–1601.
2. Herbrecht R, Denning DW, Patterson TF, Bennett JE, Greene RE, Oestmann JW, Kern WV, Marr KA, Ribaud P, Lortholary O, Sylvester R, Rubin RH, Wingard JR, Stark P, Durand C, Caillot D, Thiel E, Chandrasekar PH, Hodges MR, Schlamm HT, Troke PF, de Pauw B. Invasive Fungal Infections Group of the European Organisation for Research and Treatment of Cancer and the Global Aspergillus Study Group. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002; 347:408–415.
3. Cornely OA, Maertens J, Bresnik M, Ebrahimi R, Ullmann AJ, Bouza E, Heussel CP, Lortholary O, Rieger C, Boehme A, Aoun M, Horst HA, Thiebaut A, Ruhnke M, Reichert D, Vianelli N, Krause SW, Olavarria E, Herbrecht R. AmBiLoad Trial Study Group. Liposomal amphotericin B as initial therapy for invasive mold infection: a randomized trial comparing a high-loading dose regimen with standard dosing (AmBiLoad trial). Clin Infect Dis. 2007; 44:1289–1297.
4. Scott LJ, Simpson D. Voriconazole: a review of its use in the management of invasive fungal infections. Drugs. 2007; 67:269–298.
5. Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Segal BH, Steinbach WJ, Stevens DA, van Burik JA, Wingard JR, Patterson TF. Infectious Diseases Society of America. Treatment of aspergillosis: clinical practice guidelines of the infectious diseases society of America. Clin Infect Dis. 2008; 46:327–360.
6. Pemán J, Salavert M, Cantón E, Jarque I, Romá E, Zaragoza R, Viudes A, Gobernado M. Voriconazole in the management of nosocomial invasive fungal infections. Ther Clin Risk Manag. 2006; 2:129–158.
7. Ascioglu S, Rex JH, de Pauw B, Bennett JE, Bille J, Crokaert F, Denning DW, Donnelly JP, Edwards JE, Erjavec Z, Fiere D, Lortholary O, Maertens J, Meis JF, Patterson TF, Ritter J, Selleslag D, Shah PM, Stevens DA, Walsh TJ. Invasive Fungal Infections Cooperative Group of the European Organization for Research and Treatment of Cancer; Mycoses Study Group of the National Institute of Allergy and Infectious Diseases. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis. 2002; 34:7–14.
8. Hughes WT, Armstrong D, Bodey GP, Bow EJ, Brown AE, Calandra T, Feld R, Pizzo PA, Rolston KV, Shenep JL, Young LS. 2002 guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin Infect Dis. 2002; 34:730–751.

9. Segal BH, Herbrecht R, Stevens DA, Ostrosky-Zeichner L, Sobel J, Viscoli C, Walsh TJ, Maertens J, Patterson TF, Perfect JR, Dupont B, Wingard JR, Calandra T, Kauffman CA, Graybill JR, Baden LR, Pappas PG, Bennett JE, Kontoyiannis DP, Cordonnier C, Viviani MA, Bille J, Almyroudis NG, Wheat LJ, Graninger W, Bow EJ, Holland SM, Kullberg BJ, Dismukes WE, De Pauw BE. Defining responses to therapy and study outcomes in clinical trials of invasive fungal diseases: Mycoses Study Group and European Organization for Research and Treatment of Cancer consensus criteria. Clin Infect Dis. 2008; 47:674–683.

10. Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, Domecq C, Greenblatt DJ. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981; 30:239–245.

11. Perfect JR, Marr KA, Walsh TJ, Greenberg RN, DuPont B, de la Torre-Cisneros J, Just-Nübling G, Schlamm HT, Lutsar I, Espinel-Ingroff A, Johnson E. Voriconazole treatment for less-common, emerging, or refractory fungal infections. Clin Infect Dis. 2003; 36:1122–1131.

12. Baden LR, Katz JT, Fishman JA, Koziol C, DelVecchio A, Doran M, Rubin RH. Salvage therapy with voriconazole for invasive fungal infections in patients failing or intolerant to standard antifungal therapy. Transplantation. 2003; 76:1632–1637.

13. Denning DW, Ribaud P, Milpied N, Caillot D, Herbrecht R, Thiel E, Haas A, Ruhnke M, Lode H. Efficacy and safety of voriconazole in the treatment of acute invasive aspergillosis. Clin Infect Dis. 2002; 34:563–571.

14. Maertens J, Raad I, Petrikkos G, Boogaerts M, Selleslag D, Petersen FB, Sable CA, Kartsonis NA, Ngai A, Taylor A, Patterson TF, Denning DW, Walsh TJ. Caspofungin Salvage Aspergillosis Study Group. Efficacy and safety of caspofungin for treatment of invasive aspergillosis in patients refractory to or intolerant of conventional antifungal therapy. Clin Infect Dis. 2004; 39:1563–1571.
15. Walsh TJ, Raad I, Patterson TF, Chandrasekar P, Donowitz GR, Graybill R, Greene RE, Hachem R, Hadley S, Herbrecht R, Langston A, Louie A, Ribaud P, Segal BH, Stevens DA, van Burik JA, White CS, Corcoran G, Gogate J, Krishna G, Pedicone L, Hardalo C, Perfect JR. Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: an externally controlled trial. Clin Infect Dis. 2007; 44:2–12.

16. Dockrell DH. Salvage therapy for invasive aspergillosis. J Antimicrob Chemother. 2008; 61 Suppl 1:i41–i44.

17. Choi SM, Park SH, Lee DG, Choi JH, Yoo JH, Min WS, Shin WS, Kim CC. Efficacy and safety profile of caspofungin as a salvage therapy for invasive fungal infections in Korean patients with hematologic diseases. Infect Chemother. 2005; 37:247–254.
18. Walsh TJ, Pappas P, Winston DJ, Lazarus HM, Petersen F, Raffalli J, Yanovich S, Stiff P, Greenberg R, Donowitz G, Schuster M, Reboli A, Wingard J, Arndt C, Reinhardt J, Hadley S, Finberg R, Laverdière M, Perfect J, Garber G, Fioritoni G, Anaissie E, Lee J. National Institute of Allergy and Infectious Diseases Mycoses Study Group. Voriconazole compared with liposomal amphotericin B for empirical antifungal therapy in patients with neutropenia and persistent fever. N Eng J Med. 2002; 346:225–234.
19. Almyroudis NG, Kontoyiannis DP, Sepkowitz KA, DePauw BE, Walsh TJ, Segal BH. Issues related to the design and interpretation of clinical trials of salvage therapy for invasive mold infection. Clin Infect Dis. 2006; 43:1449–1455.

20. Kang CI, Kim SH, Park WB, Lee KD, Kim HB, Park SW, Choe YJ, Oh MD, Choe KW. Clinical manifestations and treatment outcome of invasive aspergillosis. Korean J Infect Dis. 2002; 34:160–166.
21. Nivoix Y, Velten M, Letscher-Bru V, Moghaddam A, Natarajan-Amé S, Fohrer C, Lioure B, Bilger K, Lutun P, Marcellin L, Launoy A, Freys G, Bergerat JP, Herbrecht R. Factors asso-ciated with overall and attributable mortality in invasive aspergillosis. Clin Infect Dis. 2008; 47:1176–1184.

22. Muijsers RB, Goa KL, Scott LJ. Voriconazole: in the treatment of invasive aspergillosis. Drugs. 2002; 62:2655–2664.
23. Pascual A, Calandra T, Bolay S, Buclin T, Bille J, Marchetti O. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin Infect Dis. 2008; 46:201–211.

24. Boyd AE, Modi S, Howard SJ, Moore CB, Keevil BG, Denning DW. Adverse reactions to voriconazole. Clin Infect Dis. 2004; 39:1241–1244.

25. Connelly LM. Retrospective chart reviews. Medsurg Nurs. 2008; 17:322–323.
Figure 1.
Kaplan-Meier probability of overall survival rate after initiation of VCZ therapy according to underlying disease status. Group A includes chemotherapy, HSCT and post-HSCT groups collectively. Group B is conservative care group. One patient in group B was missing 31 days after enrollment. The P value was calculated by the Log-rank test.
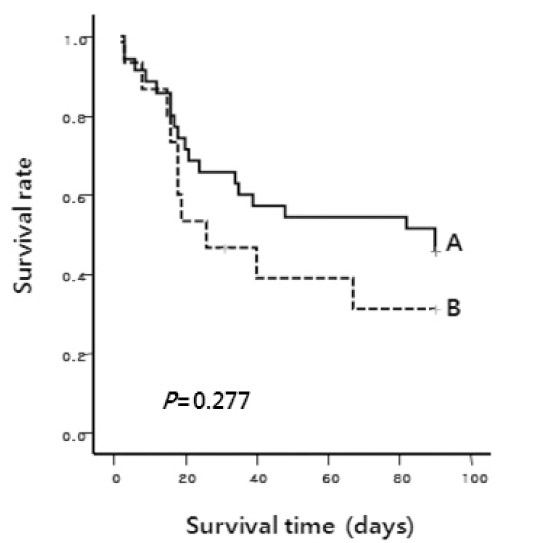
Table 1.
Baseline Demographic and Clinical Characteristics of Patients
| Characteristics | No. (%) of patients (n=50) |
|---|---|
| Sex | |
| Male | 27 (54.0) |
| Female | 23 (46.0) |
| Mean age±SD [range], years | 44.4±12.6 [15-65] |
| Underlying disease | |
| AML | 27 (54.0) |
| ALL | 8 (16.0) |
| CML | 4 (8.0) |
| MDS | 3 (6.0) |
| Lymphoma | 3 (6.0) |
| Others* | 5 (10.0) |
| Treatment group | |
| Chemotherapy group | 22 (44.0) |
| HSCT and Post-HSCT group | 13 (26.0) |
| Conservative care | 15 (30.0) |
| Classification of IA | |
| Proven | 1 (2.0) |
| Probable | 49 (98.0) |
| Site of IA | |
| Lung | 47 (94.0) |
| Sinus | 3 (6.0) |
| Response to initial antifungal therapy | |
| Refractoriness | 48 (96.0) |
| Intolerance | 2 (4.0) |
| Neutropenia at enrollment | |
| Neutropenic | 23 (46.0) |
| Non-neutropenic | 27 (54.0) |
| Duration of previous antifungal theapy | |
| Mean±SD (days) | 13.9±8.8 |
| >7 days | 35 (70.0) |
| >30 days | 3 (6.0) |
| Duration of voriconazole therapy | |
| Median (days) | 19 |
| IQR (days) | 49 |
| >90 days (%) | 10 (20.0) |
Table 2.
Overall Efficacy of Voriconazole Therapy
Table 3.
Distribution of Patients with a Favorable Response to Voriconazole Therapy at the End of Therapy
| Factors | No. of patients with favorable response/total No. of assessable patients (%) |
|---|---|
| Underlying disease | |
| AML | 10/27 (37.0) |
| ALL | 3/8 (37.5) |
| CML | 1/4 (25.0) |
| MDS | 2/3 (66.7) |
| Lymphoma | 0/3 (0.0) |
| Others* | 0/5 (0.0) |
| Treatment group | |
| Chemotherapy group | 9/22 (40.9) |
| HSCT & post-HSCT group | 5/13 (38.5) |
| Conservative care | 2/15 (13.3) |
| Classification of IA | |
| Proven | 0/1 (0.0) |
| Probable | 16/49 (32.7) |
| Site of IA | |
| Lung | 16/47 (34.0) |
| Sinus | 0/3 (0.0) |
| Response to initial antifungal therapy | |
| Refractoriness | 15/48 (31.3) |
| Intolerance | 1/2 (50.0) |
| Neutropenia at enrollment | |
| Neutropenic | 6/23 (26.1) |
| Non-neutropenic | 10/27 (37.0) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download