Abstract
Purpose
Stimulating the proliferation and migration of periodontal ligament cells (PDLCs) has become the main goal of periodontal regeneration. To accomplish this goal, regeneration procedures have been developed, but results have not been predictable. Recently, tissue engineering using enamel matrix derivatives (EMDs) and growth factors has been applied to periodontal regeneration; however, the mechanism of EMDs is largely unknown. The aim of this study was to investigate the effects of EMDs on the proliferation and release of growth factors from PDLCs.
Materials and methods
Human PDLCs were removed from individually extracted 3rd molars of healthy young adults, and cultured in the media containing EMDs (Emdogain, Biora, Malmo, Sweden) at concentration of 0, 12.5, 25, 50, 100, and 200 μ g/mL each. Cell proliferation and ALP (alkaline phosphatase) activity were measured. The evaluation of growth factors released by PDLCs was also performed by one-way analysis of variance (ANOVA) and Bonferroni's multiple comparison test.
Results
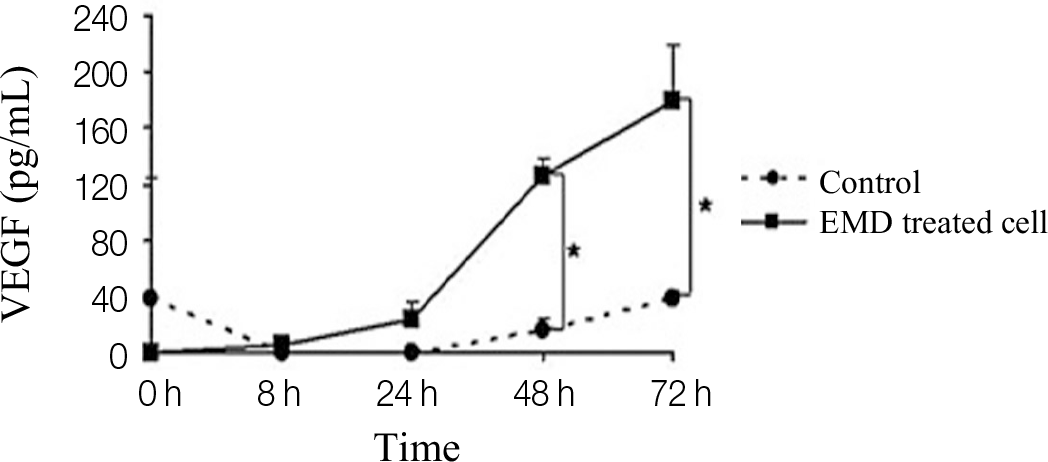
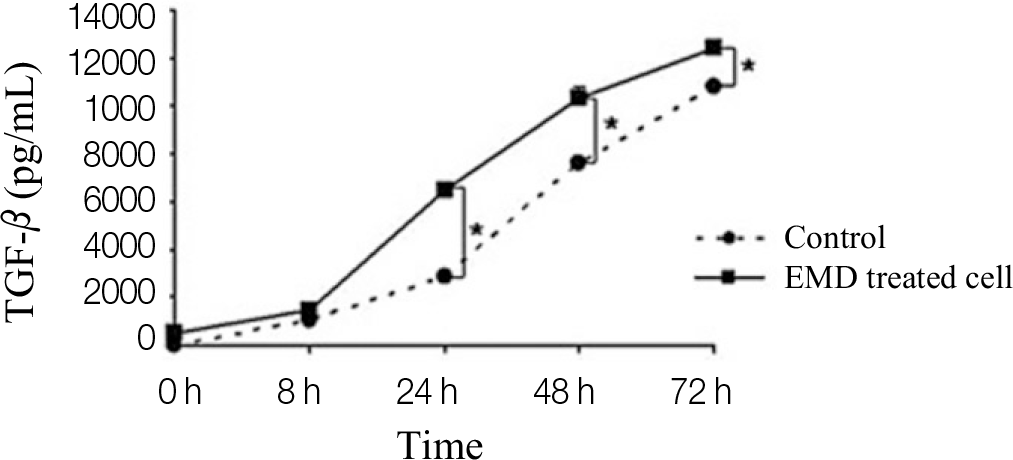
Significantly increased proliferation and ALP activity were observed in PDLCs treated with over 25 μ g/mL and 50 μ g/mL EMDs, respectively. Additionally, treatment of PDLCs with 50 μ g/mL resulted in significantly increased release of vascular endothelial growth factor (VEGF) and transforming growth factor (TGF)-β after 24 h and 48 h, respectively.
Go to : 
REFERENCES
1.Hoang AM., Oates TW., Cochran DL. In vitro wound healing responses to enamel matrix derivative. J Periodontol. 2000. 71:1270–7.

2.Chong CH., Carnes DL., Moritz AJ., Oates T., Ryu OH., Simmer J., Cochran DL. Human periodontal fibroblast response to enamel matrix derivative, amelogenin, and platelet-derived growth factor-BB. J Periodontol. 2006. 77:1242–52.

3.Slavkin HC. Towards a cellular and molecular understanding of periodontics. Cementogenesis revisited. J Periodontol. 1976. 47:249–55.

4.Gestrelius S., Andersson C., Lidström D., Hammarström L., Somerman M. In vitro studies on periodontal ligament cells and enamel matrix derivative. J Clin Periodontol. 1997. 24:685–92.

5.Hammarström L. Enamel matrix, cementum development and regeneration. J Clin Periodontol. 1997. 24:658–68.

6.Heijl L., Heden G., Svärdström G., Ostgren A. Enamel matrix derivative (EMDOGAIN) in the treatment of intrabony periodontal defects. J Clin Periodontol. 1997. 24:705–14.

7.Froum SJ., Weinberg MA., Rosenberg E., Tarnow D. A comparative study utilizing open flap debridement with and without enamel matrix derivative in the treatment of periodontal intrabony defects: a 12-month re-entry study. J Periodontol. 2001. 72:25–34.

8.Sculean A., Donos N., Miliauskaite A., Arweiler N., Brecx M. Treatment of intrabony defects with enamel matrix proteins or bioabsorbable membranes. A 4-year follow-up split-mouth study. J Periodontol. 2001. 72:1695–701.

9.Van der Pauw MT., Van den Bos T., Everts V., Beertsen W. Enamel matrix-derived protein stimulates attachment of periodontal ligament fibroblasts and enhances alkaline phosphatase activity and transforming growth factor beta1 release of periodontal ligament and gingival fibroblasts. J Periodontol. 2000. 71:31–43.
11.Boyan LA., Bhargava G., Nishimura F., Orman R., Price R., Terranova VP. Mitogenic and chemotactic responses of human periodontal ligament cells to the different isoforms of platelet-derived growth factor. J Dent Res. 1994. 73:1593–600.

12.Lyngstadaas SP., Lundberg E., Ekdahl H., Andersson C., Gestrelius S. Autocrine growth factors in human periodontal ligament cells cultured on enamel matrix derivative. J Clin Periodontol. 2001. 28:181–8.

13.Grandin HM., Gemperli AC., Dard M. Enamel matrix derivative: a review of cellular effects in vitro and a model of molecular arrangement and functioning. Tissue Eng Part B Rev. 2012. 18:181–202.
14.Schlueter SR., Carnes DL., Cochran DL. In vitro effects of enamel matrix derivative on microvascular cells. J Periodontol. 2007. 78:141–51.

15.Mirastschijski U., Konrad D., Lundberg E., Lyngstadaas SP., Jorgensen LN., Agren MS. Effects of a topical enamel matrix derivative on skin wound healing. Wound Repair Regen. 2004. 12:100–8.
16.Pfeilschifter J., Oechsner M., Naumann A., Gronwald RG., Minne HW., Ziegler R. Stimulation of bone matrix apposition in vitro by local growth factors: a comparison between insulin-like growth factor I, platelet-derived growth factor, and transforming growth factor beta. Endocrinology. 1990. 127:69–75.
Go to : 
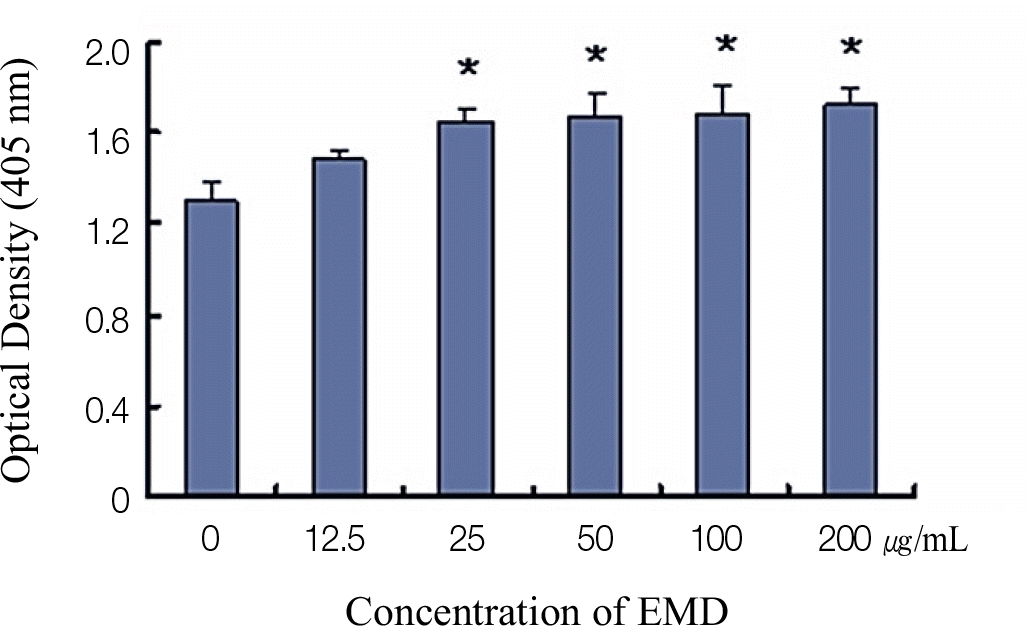 | Fig. 1.The effect of different enamel matrix derivatives (EMDs) concentrations on the proliferation of PDLCs. The results represent the means ± SD of three replicates. ∗ Significantly different from the control group (P < .05). |
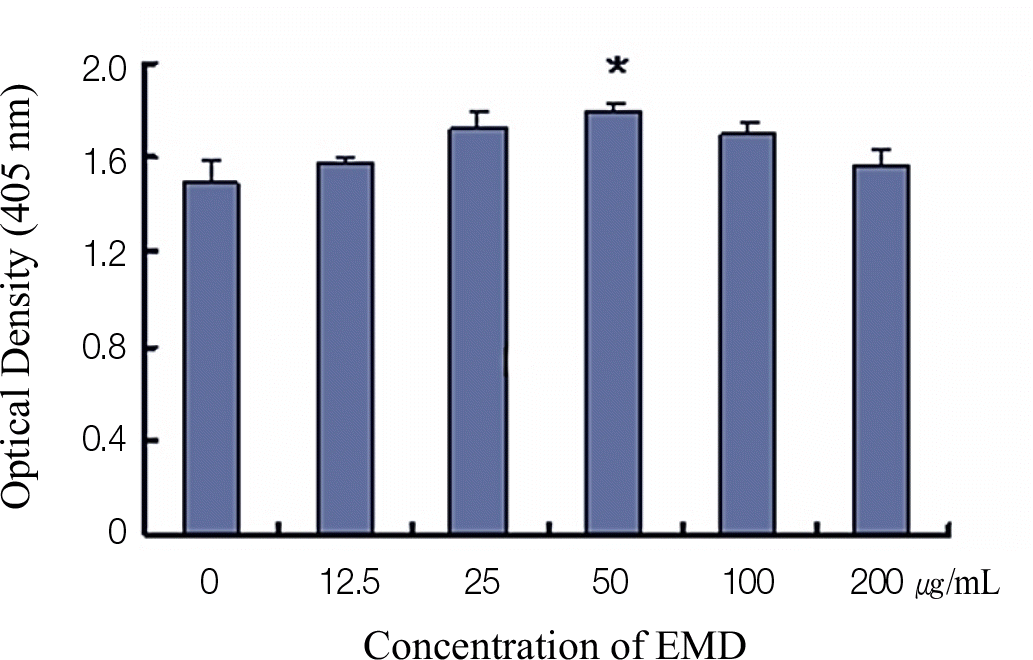 | Fig. 2.The effects of different EMDs concentrations on the ALP activity of PDLCs. The results represent the means ± SD of three replicates. ∗ Significantly different from the control group (P < .05). |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download