Abstract
Purpose
The purpose of this study is to identify the effect of mesenchymal stem cell proliferation on recombinant human transforming growth factor-beta (rhTGF-β 2) / poly (D,L-lactide-co-glycolide) (PLGA) treated titanium discs by electrospray.
Materials and methods
Anodized titanium surface coated with PLGA was used for a control group to compare anodized titanium surface coated with 125 ng/ml and 500 ng/ml rhTGF-β 2 as test groups. Atomic force microscope (AFM) test was utilized to determine the difference in coating surface roughness, and field-emission scanning electron microscopy (FE-SEM) was taken to visualize even distribution of coating particles on titanium discs. The mesenchymal stem cell proliferation was tested by using MTT (3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl-tetrazolium bromide) assay on 1st, 4th, 7th days.
Results
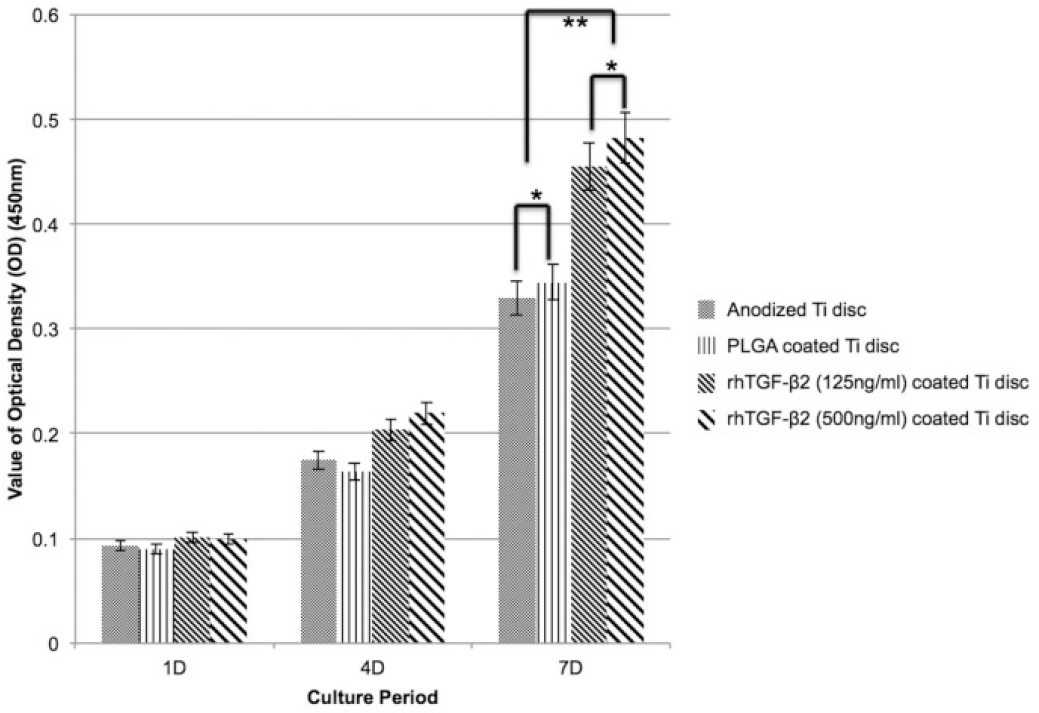
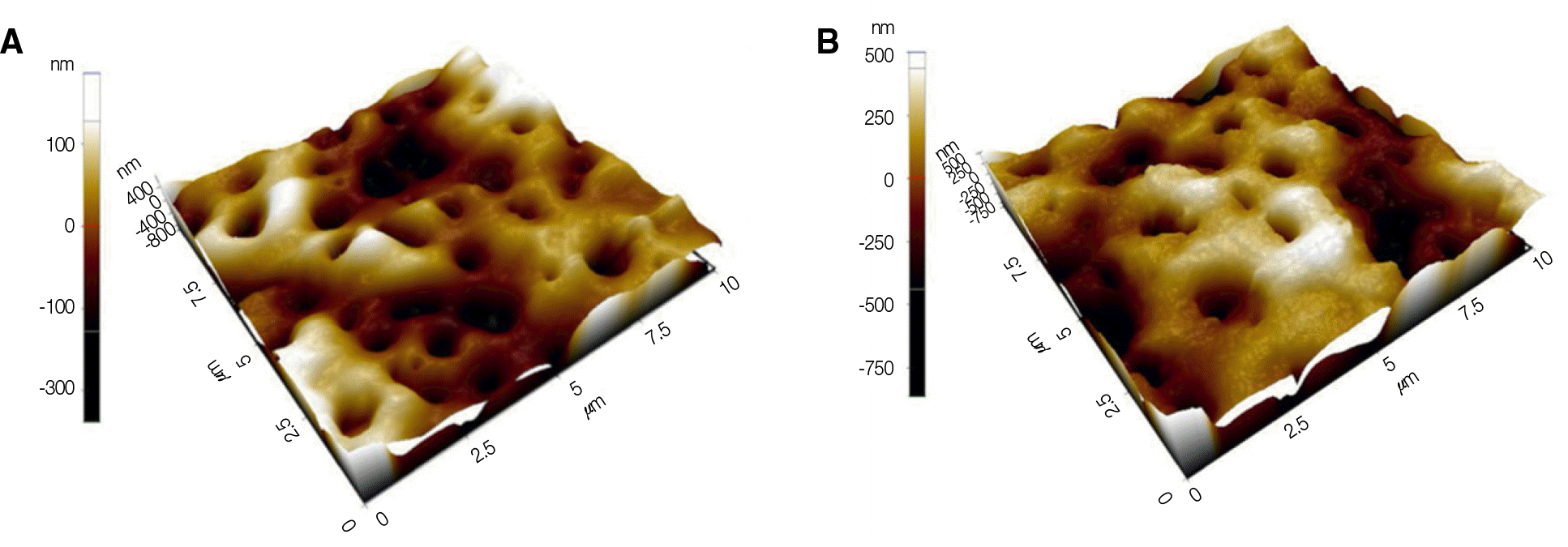
According to AFM results, there was no statistically significant difference in titanium discs treated with PLGA and with rhTGF-β 2/PLGA (P>.05). MTT assay test results showed that there was statistically significant difference in mesenchymal stem cell proliferation on test groups compared to control groups at 7th day, and cell viability on discs coated with rhTGF-β 2 was significantly higher than control groups (P<.05).
Go to : 
REFERENCES
1.Hermann JS., Cochran DL., Nummikoski PV., Buser D. Crestal bone changes around titanium implants. A radiographic evaluation of unloaded nonsubmerged and submerged implants in the canine mandible. J Periodontol. 1997. 68:1117–30.

2.Sul YT., Johansson CB., Petronis S., Krozer A., Jeong Y., Wennerberg A., Albrektsson T. Characteristics of the surface oxides on turned and electrochemically oxidized pure titanium implants up to dielectric breakdown: the oxide thickness, micropore configurations, surface roughness, crystal structure and chemical composition. Biomaterials. 2002. 23:491–501.
3.Xie J., Baumann MJ., McCabe LR. Osteoblasts respond to hydroxyapatite surfaces with immediate changes in gene expression. J Biomed Mater Res A. 2004. 71:108–17.

4.Ivanoff CJ., Hallgren C., Widmark G., Sennerby L., Wennerberg A. Histologic evaluation of the bone integration of TiO(2) blasted and turned titanium microimplants in humans. Clin Oral Implants Res. 2001. 12:128–34.

5.Orsini G., Assenza B., Scarano A., Piattelli M., Piattelli A. Surface analysis of machined versus sandblasted and acid-etched titanium implants. Int J Oral Maxillofac Implants. 2000. 15:779–84.
6.Junker R., Dimakis A., Thoneick M., Jansen JA. Effects of implant surface coatings and composition on bone integration: a systematic review. Clin Oral Implants Res. 2009. 20:185–206.

7.Bosetti M., Boccafoschi F., Leigheb M., Cannas MF. Effect of different growth factors on human osteoblasts activities: a possible application in bone regeneration for tissue engineering. Biomol Eng. 2007. 24:613–8.

8.Kim HD., Valentini RF. Human osteoblast response in vitro to platelet-derived growth factor and transforming growth factor-beta delivered from controlled-release polymer rods. Biomaterials. 1997. 18:1175–84.
9.De Ranieri A., Virdi AS., Kuroda S., Shott S., Leven RM., Hallab NJ., Sumner DR. Local application of rhTGF-beta2 enhances peri-implant bone volume and bone-implant contact in a rat model. Bone. 2005. 37:55–62.
10.Cho YJ., Heo SJ., Koak JY. Kim SK, Lee JH. Cellular responses on anodized titanium discs coated with 1α,25-dihydroxyvit-amin D3 incorporated Poly(D,L-lactide-co-glycolide) (PLGA) nanoparticles. J Korean Acad Prosthodont. 2008. 46:620–7.
11.Lee SY. Effect of poly(D,L-lactide-co-glycolide)/bone morphogenic protein-2 coating of anodized titanium surface on osteoblast-like cells. MS Thesis. In: Korea, Seoul University,. 2010.
12.Fan H., Tao H., Wu Y., Hu Y., Yan Y., Luo Z. TGF-β 3 immobilized PLGA-gelatin/chondroitin sulfate/hyaluronic acid hybrid scaffold for cartilage regeneration. J Biomed Mater Res A. 2010. 95:982–92.
13.Freiberg S., Zhu XX. Polymer microspheres for controlled drug release. Int J Pharm. 2004. 282:1–18.

14.Sims NA., Gooi JH. Bone remodeling: Multiple cellular interactions required for coupling of bone formation and resorption. Semin Cell Dev Biol. 2008. 19:444–51.

15.Centrella M., McCarthy TL., Canalis E. Transforming growth factor-beta and remodeling of bone. J Bone Joint Surg Am. 1991. 73:1418–28.

16.Centrella M., McCarthy TL., Canalis E. Transforming growth factor beta is a bifunctional regulator of replication and collagen synthesis in osteoblast-enriched cell cultures from fetal rat bone. J Biol Chem. 1987. 262:2869–74.

17.Lomri A., Marie PJ. Bone cell responsiveness to transforming growth factor beta, parathyroid hormone, and prostaglandin E2 in normal and postmenopausal osteoporotic women. J Bone Miner Res. 1990. 5:1149–55.
18.Lomri A., Marie PJ. Effects of transforming growth factor type beta on expression of cytoskeletal proteins in endosteal mouse osteoblastic cells. Bone. 1990. 11:445–51.
19.Machwate M., Jullienne A., Moukhtar M., Lomri A., Marie PJ. cfos protooncogene is involved in the mitogenic effect of transforming growth factor-beta in osteoblastic cells. Mol Endocrinol. 1995. 9:187–98.

20.Robey PG., Young MF., Flanders KC., Roche NS., Kondaiah P., Reddi AH., Termine JD., Sporn MB., Roberts AB. Osteoblasts synthesize and respond to transforming growth factor-type beta (TGF-beta) in vitro. J Cell Biol. 1987. 105:457–63.

21.Rosen DM., Stempien SA., Thompson AY., Seyedin SM. Transforming growth factor-beta modulates the expression of osteoblast and chondroblast phenotypes in vitro. J Cell Physiol. 1988. 134:337–46.

22.Sena K., Sumner DR., Virdi AS. Effect of recombinant human transforming growth factor-beta2 dose on bone formation in rat femur titanium implant model. J Biomed Mater Res A. 2010. 92:1210–7.
23.Yoo SY., Kim SK., Heo SJ., Koak JY., Lee JH., Park JM. Biochemical responses of anodized titanium implants with a poly(lactide-co-glycolide)/bone morphogenic protein-2 submicron particle coating. Part 1: an in vitro study. Int J Oral Maxillofac Implants. 2015. 30:512–8.

24.Agrawal CM., Niederauer GG., Athanasiou KA. Fabrication and characterization of PLA-PGA orthopedic implants. Tissue Eng. 1995. 1:241–52.

25.Hahn H., Palich W. Preliminary evaluation of porous metal surfaced titanium for orthopedic implants. J Biomed Mater Res. 1970. 4:571–7.

26.Geesink RG., de Groot K., Klein CP. Bonding of bone to apatite-coated implants. J Bone Joint Surg Br. 1988. 70:17–22.

27.de Groot K., Gesink R., Klein CP. Plasma sprayed coatings of hydroxylapatite. J Biomed Mater Res. 1987. 21:1375–81.

Go to : 
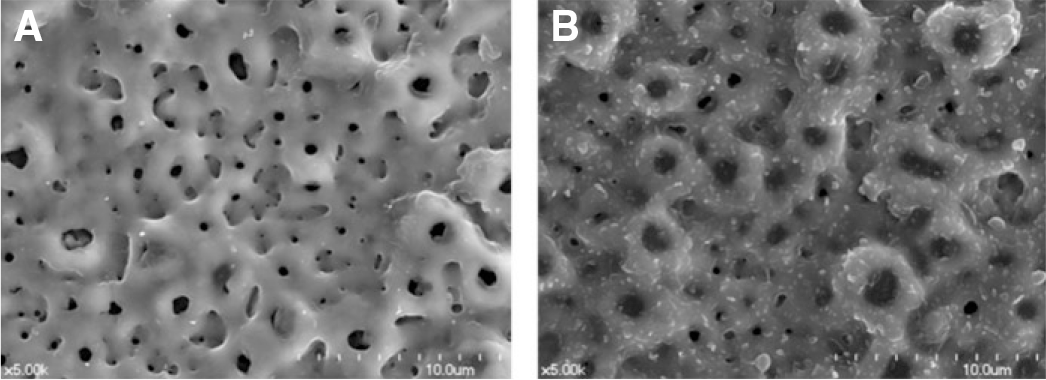 | Fig. 1.SEM image of anodized titanium implant coated by electrospray (×5,000). (A) Anodized Ti disc, (B) Anodized Ti disc coated with 40 ㎕rhTGF-β 2 (500 ng/ml) submicron particles by electrospray technique. |
 | Fig. 2.The results of surface roughness measured by AFM (10 ㎛× 10 ㎛). (A) Anodized titanium disc, (B) Anodized titanium disc coated with 40 ㎕rhTGF-β 2 (500 ng/ml per disc). |
 | Fig. 3.MTT assay results. The result presents that there was tendency of cell proliferation increasing by time, however; there was no statistically significant difference among groups in 1 day and 4 days. At the 7 days of cell culture, there was statistically significant difference between control groups and experimental groups (∗P<.05, ∗∗P<.05) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download