Abstract
Purpose
This preliminary rabbit study was conducted to evaluate the effect of recombinant human transforming growth factor-β 2 (rhTGF-β 2)/poly lactic-co-glycolic acid (PLGA) coating on osseointegration of the titanium (Ti) implant.
Materials and methods
Eight Ti implants were anodized with 300 voltages for three minutes. Four of those were coated with rhTGF-β 2/PLGA by an electrospray method as the experimental group. The implants were placed into tibiae of four New Zealand rabbits, two implants per a tibia, one implant per each group. After 3 and 6 weeks, every two rabbits were sacrificed and micro-computed tomography (microCT) was taken for histomorphometric analysis.
Results
In scanning electron microscope (SEM) image, the surface of rhTGF-β 2/PLGA coated Ti implant showed well distributed particles. Although statistically insignif-icant, microCT analysis showed that experimental group has higher bone volume / total volume (BV/TV) and trabecular thickness (Tb.Th) values relatively. Cross sectional view also showed more newly formed bone in the experimental group.
Go to : 
REFERENCES
1. Hermann JS, Cochran DL, Nummikoski PV, Buser D. Crestal bone changes around titanium implants. A radiographic evaluation of unloaded nonsubmerged and submerged implants in the ca-nine mandible. J Periodontol. 1997; 68:1117–30.

2. Sul YT, Johansson CB, Petronis S, Krozer A, Jeong Y, Wennerberg A, Albrektsson T. Characteristics of the surface oxides on turned and electrochemically oxidized pure titanium implants up to dielectric breakdown: the oxide thickness, micropore con-figurations, surface roughness, crystal structure and chemical composition. Biomaterials. 2002; 23:491–501.
3. Xie J, Baumann MJ, McCabe LR. Osteoblasts respond to hy-droxyapatite surfaces with immediate changes in gene expression. J Biomed Mater Res A. 2004; 71:108–17.

4. Ivanoff CJ, Hallgren C, Widmark G, Sennerby L, Wennerberg A. Histologic evaluation of the bone integration of TiO(2) blasted and turned titanium microimplants in humans. Clin Oral Implants Res. 2001; 12:128–34.

5. Orsini G, Assenza B, Scarano A, Piattelli M, Piattelli A. Surface analysis of machined versus sandblasted and acid-etched titanium implants. Int J Oral Maxillofac Implants. 2000; 15:779–84.
6. Huang HH, Ho CT, Lee TH, Lee TL, Liao KK, Chen FL. Effect of surface roughness of ground titanium on initial cell adhesion. Biomol Eng. 2004; 21:93–7.

7. Junker R, Dimakis A, Thoneick M, Jansen JA. Effects of implant surface coatings and composition on bone integration: a systematic review. Clin Oral Implants Res. 2009; 20:185–206.

8. Bosetti M, Boccafoschi F, Leigheb M, Cannas MF. Effect of different growth factors on human osteoblasts activities: a possible application in bone regeneration for tissue engineering. Biomol Eng. 2007; 24:613–8.

9. Kim HD, Valentini RF. Human osteoblast response in vitro to platelet-derived growth factor and transforming growth factor-beta delivered from controlled-release polymer rods. Biomaterials. 1997; 18:1175–84.
10. De Ranieri A, Virdi AS, Kuroda S, Shott S, Leven RM, Hallab NJ, Sumner DR. Local application of rhTGF-beta2 enhances peri-implant bone volume and bone-implant contact in a rat model. Bone. 2005; 37:55–62.
11. Lee SY. Effect of poly(D, L-lactide-co-glycolide)/bone mor-phogenic protein-2 coating of anodized titanium surface on osteoblast-like cells. MS Thesis. In: Korea, Seoul University. 2010.
12. Fan H, Tao H, Wu Y, Hu Y, Yan Y, Luo Z. TGF-β 3 immobilized PLGA-gelatin/chondroitin sulfate/hyaluronic acid hybrid scaf-fold for cartilage regeneration. J Biomed Mater Res A. 2010; 95:982–92.
13. Woosley JP, Turnbull RJ, Kim K. Field injection electrostatic spraying of liquid hydrogen. J Appl Phys. 1988; 64:4278–84.

14. Kim K, Turnbull RJ. Generation of charged drops of insulating liquids by electrostatic spraying. J Appl Phys. 1976; 47:1964–9.

15. Park IP, Kim SK, Lee SJ, Lee JH. The relationship between initial implant stability quotient values and bone-to-implant contact ratio in the rabbit tibia. J Adv Prosthodont. 2011; 3:76–80.

16. Xu B, Zhang J, Brewer E, Tu Q, Yu L, Tang J, Krebsbach P, Wieland M, Chen J. Osterix enhances BMSC-associated osseointegration of implants. J Dent Res. 2009; 88:1003–7.
17. Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Mu¨ller R. Guidelines for assessment of bone mi-crostructure in rodents using micro-computed tomography. J Bone Miner Res. 2010; 25:1468–86.

18. Sims NA, Gooi JH. Bone remodeling: Multiple cellular interactions required for coupling of bone formation and resorption. Semin Cell Dev Biol. 2008; 19:444–51.

19. Centrella M, McCarthy TL, Canalis E. Current concepts review: transforming growth factor-beta and remodeling of bone. J Bone Joint Surg. 1991; 73A:1418–28.
20. Centrella M, McCarthy TL, Canalis E. Transforming growth factor beta is a bifunctional regulator of replication and collagen syn-thesis in osteoblast-enriched cell cultures from fetal rat bone. J Biol Chem. 1987; 262:2869–74.

21. Lomri A, Marie PJ. Bone cell responsiveness to transforming growth factor beta, parathyroid hormone, and prostaglandin E2 in normal and postmenopausal osteoporotic women. J Bone Miner Res. 1990; 5:1149–55.
22. Lomri A, Marie PJ. Effects of transforming growth factor type beta on expression of cytoskeletal proteins in endosteal mouse osteoblastic cells. Bone. 1990; 11:445–51.
23. Machwate M, Jullienne A, Moukhtar M, Lomri A, Marie PJ. c-fos protooncogene is involved in the mitogenic effect of transforming growth factor-beta in osteoblastic cells. Mol Endocrinol. 1995; 9:187–98.

24. Robey PG, Young MF, Flanders KC, Roche NS, Kondaiah P, Reddi AH, Termine JD, Sporn MB, Roberts AB. Osteoblasts syn-thesize and respond to transforming growth factor-type beta (TGF-beta) in vitro. J Cell Biol. 1987; 105:457–63.

25. Rosen DM, Stempien SA, Thompson AY, Seyedin SM. Transforming growth factor-beta modulates the expression of osteoblast and chondroblast phenotypes in vitro. J Cell Physiol. 1988; 134:337–46.

26. Sena K, Sumner DR, Virdi AS. Effect of recombinant human transforming growth factor-beta2 dose on bone formation in rat femur titanium implant model. J Biomed Mater Res A. 2010; 92:1210–7.
27. Agrawal CM, Niederauer GG, Athanasiou KA. Fabrication and Characterization of PLA-PGA Orthopedic Implants. Tissue Eng. 1995; 1:241–52.

28. Yoo SY, Kim SK, Heo SJ, Koak JY, Lee JH, Park YK, Kim E. A study of mesenchymal stem cell proliferation and surface characteristics of the titanium discs coated with MS275/PLGA by an electrospray. J Korean Acad Prosthodont. 2012; 50:285–91.

Go to : 
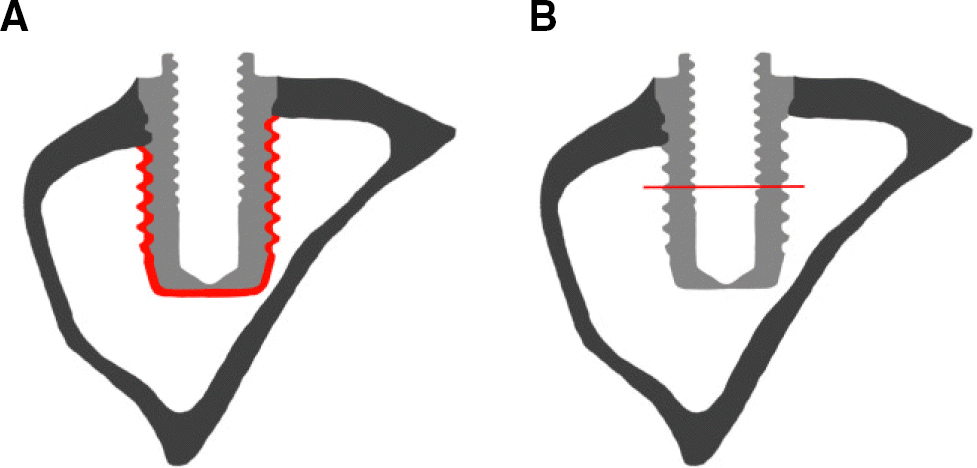 | Fig. 1.Schematic image of implant. (A) Region of interest (ROI) for microCT,(B) reference for cross sectional view. |
 | Fig. 2.SEM image of implant surface (×5,000). (A) Control group (implant without surface coating), (B) Experimental group (implant with rhTGF-β 2/PLGA coating via electrospray method). |
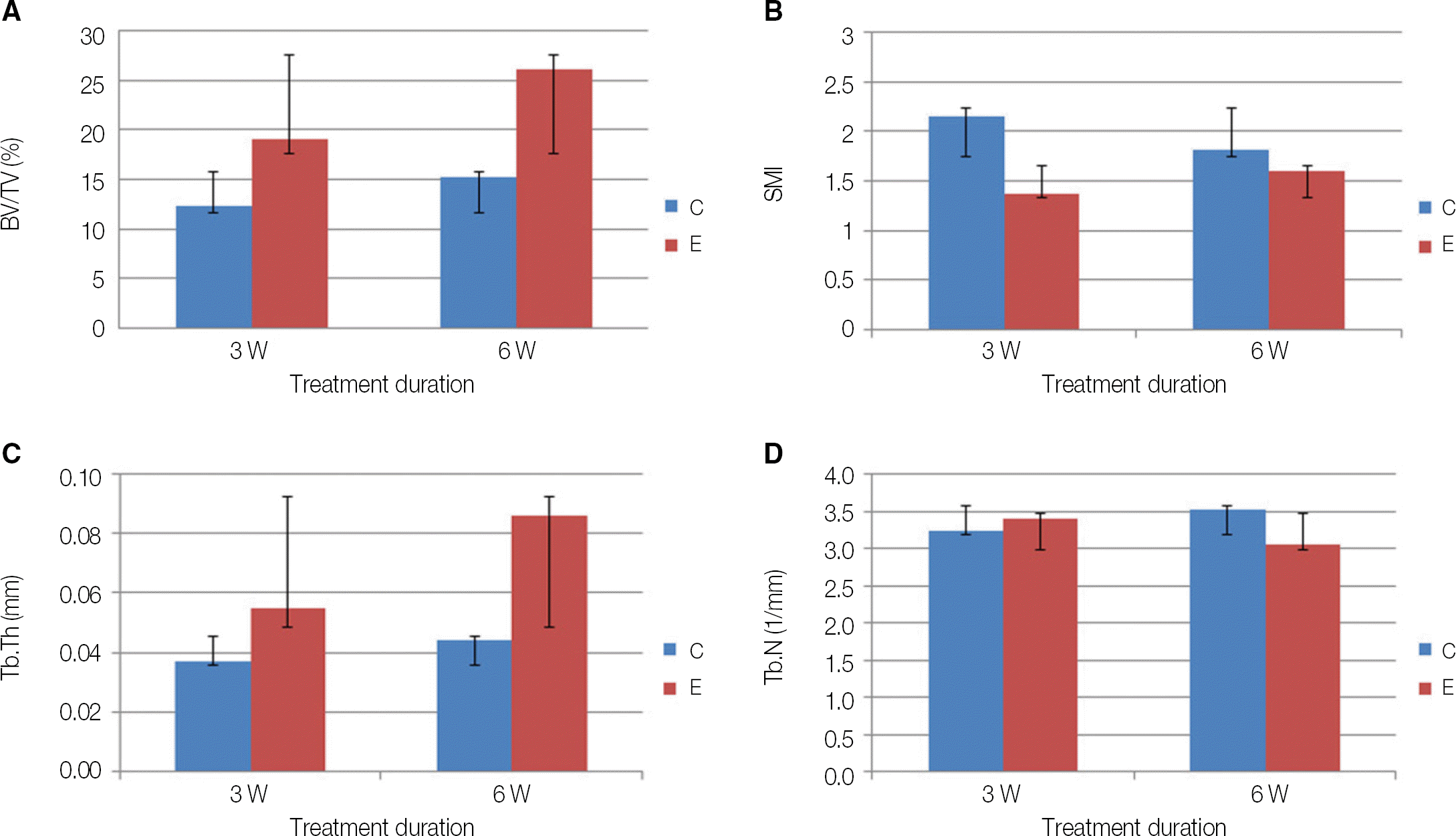 | Fig. 3.MicroCT analysis. (A) BV/TV (bone volume per total volume), (B) SMI (structure model index), (C) Tb.Th (trabecular thickness), (D) Tb.N (trabecular number), C (control), E (experimental) (P>.05). |
 | Fig. 4.Cross sectional view of microCT. (A) 3 weeks control, (B) 3 weeks experimental (rhTGF-β 2/ PLGA coated implant), (C) 6 weeks control, (D) 6 weeks experimental (rhTGF-β 2/ PLGA coated implant). |
Table 1.
MicroCT analysis, mean (SD)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download