Abstract
Purpose
The purpose of this study was to evaluate the surface characteristics and response of osteoblast-like cell at SLA surface treated with NaOH.
Materials and methods
Three kinds of specimens were fabricated for the experiment groups. Control group was a machined surface, SLA group was a conventionally SLA treated surface, and SLA/NaOH gorup was SLA surface treated with NaOH. To evaluate the surface characteristics, the surface elemental composition (XPS), surface roughness and surface contact angle were evaluated in each group. And the cytotoxicity, cell adhesion, cell proliferation and ATP activity of osteoblast-like cells (MG-63 cells) were compared in each group for evaluatation of the cell responses. Statistical comparisons between groups were carried out via one-way ANOVA using the SPSS software (SPSS Inc., Chicago, USA), and then performed multiple comparisons. The differences were considered statistically significant at P<.05.
Results
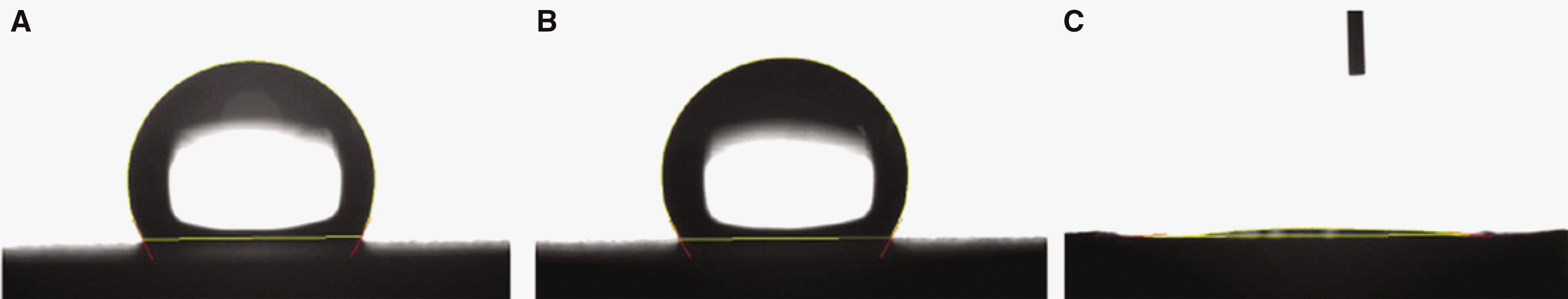
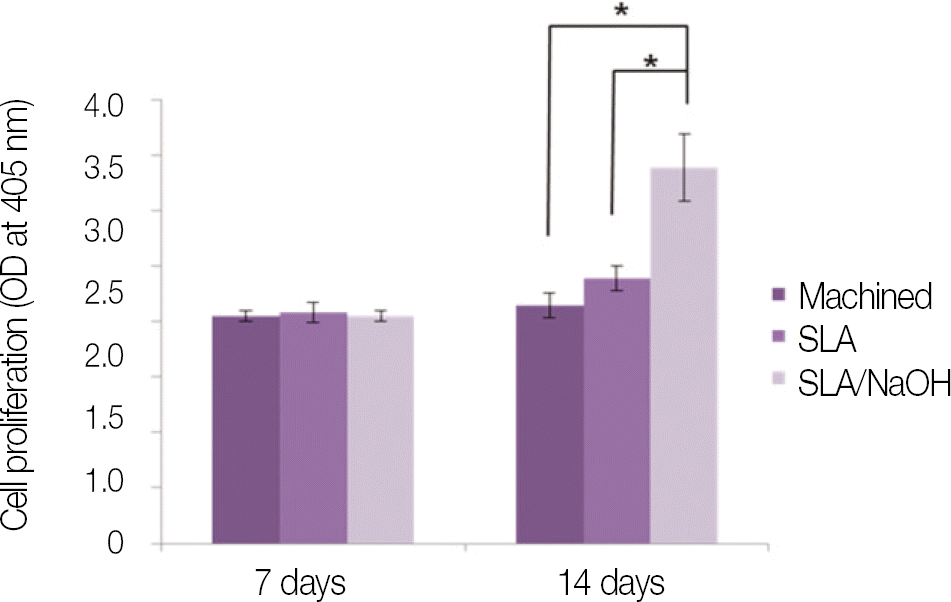
SLA surface treated with NaOH (SLA / NaOH group) was changed to hydrophilic surface. All groups did not show the cytotoxicity to the MG-63. In cell adhesion studies, SLA / NaOH group showed the higher degree of adhesion than anothers (P<.05), Up to 7 days of incubation, the proliferation was showed the increasing tendency in all groups but SLA / NaOH group showed the highest cell proliferation between the three groups (P<.05). At 7 days of incubation, there was no difference in ALP activities between the three groups, but at 14 days, SLA / NaOH group showed significant increase in ALP activities (P<.05).
Go to : 
REFERENCES
1. Bra�nemark PI, Adell R, Breine U, Hansson BO, Lindstro¨m J, Ohlsson A. Intra-osseous anchorage of dental prostheses. I. Experimental studies. Scand J Plast Reconstr Surg. 1969; 3:81–100.
2. Adell R, Lekholm U, Rockler B, Bra�nemark PI. A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int J Oral Surg. 1981; 10:387–416.

3. Lindquist LW, Carlsson GE, Jemt T. A prospective 15-year follow-up study of mandibular fixed prostheses supported by osseointegrated implants. Clinical results and marginal bone loss. Clin Oral Implants Res. 1996; 7:329–36.

4. Lekholm U, Gunne J, Henry P, Higuchi K, Linde′n U, Bergstro¨m C, van Steenberghe D. Survival of the Bra�nemark implant in partially edentulous jaws: a 10-year prospective multicenter study. Int J Oral Maxillofac Implants. 1999; 14:639–45.
5. Brocard D, Barthet P, Baysse E, Duffort JF, Eller P, Justumus P, Marin P, Oscaby F, Simonet T, Benque′ E, Brunel G. A multicenter report on 1, 022 consecutively placed ITI implants: a 7-year longitudinal study. Int J Oral Maxillofac Implants. 2000; 15:691–700.
6. Jemt T, Johansson J. Implant treatment in the edentulous maxillae: a 15-year follow-up study on 76 consecutive patients provided with fixed prostheses. Clin Implant Dent Relat Res. 2006; 8:61–9.

7. Albrektsson T, Bra�nemark PI, Hansson HA, Lindstro¨m J. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop Scand. 1981; 52:155–70.
8. Hutton JE, Heath MR, Chai JY, Harnett J, Jemt T, Johns RB, McKenna S, McNamara DC, van Steenberghe D, Taylor R, et al. Factors related to success and failure rates at 3-year fol-low-up in a multicenter study of overdentures supported by Bra�nemark implants. Int J Oral Maxillofac Implants. 1995; 10:33–42.
9. Jaffin RA, Berman CL. The excessive loss of Bra�nemark fixtures in type IV bone: a 5-year analysis. J Periodontol. 1991; 62:2–4.

10. Sullivan DY, Sherwood RL, Mai TN. Preliminary results of a multicenter study evaluating a chemically enhanced surface for machined commercially pure titanium implants. J Prosthet Dent. 1997; 78:379–86.

11. Le Gue′hennec L, Soueidan A, Layrolle P, Amouriq Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent Mater. 2007; 23:844–54.
12. Lee YJ, Lee BU, Kim YS. Current studies of implant surface treatment - in perspective of bone healing mechanism. Implantology. 2003; 12:12–29.
13. Urban RM, Jacobs JJ, Tomlinson MJ, Gavrilovic J, Black J, Peoc'h M. Dissemination of wear particles to the liver, spleen, and abdominal lymph nodes of patients with hip or knee replacement. J Bone Joint Surg Am. 2000; 82:457–76.

14. Song HJ. Advanced surface modification techniques for enhancing osseointegration of titanium implant. J Korean Dent Assoc. 2010; 48:97–105.
15. Sanz A, Oyarzun A, Farias D, Diaz I. Experimental study of bone response to a new surface treatment of endosseous titanium implants. Implant Dent. 2001; 10:126–31.
16. Piattelli M, Scarano A, Paolantonio M, Iezzi G, Petrone G, Piattelli A. Bone response to machined and resorbable blast material titanium implants: an experimental study in rabbits. J Oral Implantol. 2002; 28:2–8.

17. Lee HJ, Song KY, Yoon TH. Effect of different surface treatments to increase biocompatibility of dental implant. J Korean Acad Prosthodont. 2006; 44:594–605.
18. Buser D, Broggini N, Wieland M, Schenk RK, Denzer AJ, Cochran DL, Hoffmann B, Lussi A, Steinemann SG. Enhanced bone apposition to a chemically modified SLA titanium surface. J Dent Res. 2004; 83:529–33.

19. Buser D, Schenk RK, Steinemann S, Fiorellini JP, Fox CH, Stich H. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J Biomed Mater Res. 1991; 25:889–902.

20. Bornstein MM, Schmid B, Belser UC, Lussi A, Buser D. Early loading of non-submerged titanium implants with a sandblast-ed and acid-etched surface. 5-year results of a prospective study in partially edentulous patients. Clin Oral Implants Res. 2005; 16:631–8.
21. Sul YT, Johansson CB, Albrektsson T. Oxidized titanium screws coated with calcium ions and their performance in rab-bit bone. Int J Oral Maxillofac Implants. 2002; 17:625–34.
22. Sul YT, Johansson CB, Jeong Y, Ro¨ser K, Wennerberg A, Albrektsson T. Oxidized implants and their influence on the bone response. J Mater Sci Mater Med. 2001; 12:1025–31.
23. Kokubo T, Kim HM, Kawashita M, Nakamura T. Bioactive metals: preparation and properties. J Mater Sci Mater Med. 2004; 15:99–107.
24. Kilpadi DV, Lemons JE. Surface energy characterization of unalloyed titanium implants. J Biomed Mater Res. 1994; 28:1419–25.

25. MacDonald DE, Deo N, Markovic B, Stranick M, Somasundaran P. Adsorption and dissolution behavior of human plasma fibronectin on thermally and chemically modified titanium dioxide particles. Biomaterials. 2002; 23:1269–79.

26. Rupp F, Scheideler L, Rehbein D, Axmann D, Geis-Gerstorfer J. Roughness induced dynamic changes of wettability of acid etched titanium implant modifications. Biomaterials. 2004; 25:1429–38.

27. Lim YJ, Oshida Y, Andres CJ, Barco MT. Surface characterizations of variously treated titanium materials. Int J Oral Maxillofac Implants. 2001; 16:333–42.
28. Yanagisawa I, Sakuma H, Shimura M, Wakamatsu Y, Yanagisawa S, Sairenji E. Effects of "wettability" of biomaterials on culture cells. J Oral Implantol. 1989; 15:168–77.
29. Smith DC, Pilliar RM, Chernecky R. Dental implant materials. I. Some effects of preparative procedures on surface topography. J Biomed Mater Res. 1991; 25:1045–68.

30. MacDonald DE, Rapuano BE, Deo N, Stranick M, Somasundaran P, Boskey AL. Thermal and chemical modification of titanium-aluminum-vanadium implant materials: effects on surface properties, glycoprotein adsorption, and MG63 cell attachment. Biomaterials. 2004; 25:3135–46.

31. Rupp F, Scheideler L, Olshanska N, de Wild M, Wieland M, Geis-Gerstorfer J. Enhancing surface free energy and hydrophilicity through chemical modification of microstructured titanium implant surfaces. J Biomed Mater Res A. 2006; 76:323–34.

32. Kim C, Kendall MR, Miller MA, Long CL, Larson PR, Humphrey MB, Madden AS, Tas AC. Comparison of titanium soaked in 5 M NaOH or 5 M KOH solutions. Mater Sci Eng C Mater Biol Appl. 2013; 33:327–339.
33. Gotfredsen K, Wennerberg A, Johansson C, Skovgaard LT, Hj � rting-Hansen E. Anchorage of TiO2-blasted, HA-coated, and machined implants: an experimental study with rabbits. J Biomed Mater Res. 1995; 29:1223–31.
34. Wennerberg A, Albrektsson T, Andersson B, Krol JJ. A histomorphometric and removal torque study of screw-shaped titanium implants with three different surface topographies. Clin Oral Implants Res. 1995; 6:24–30.
36. Brett PM, Harle J, Salih V, Mihoc R, Olsen I, Jones FH, Tonetti M. Roughness response genes in osteoblasts. Bone. 2004; 35:124–33.

37. Wennerberg A, Albrektsson T, Ulrich H, Krol JJ. An optical three-dimensional technique for topographical descriptions of surgi-cal implants. J Biomed Eng. 1992; 14:412–8.

38. Kilpadi DV, Lemons JE. Surface energy characterization of unalloyed titanium implants. J Biomed Mater Res. 1994; 28:1419–25.

39. MacDonald DE, Deo N, Markovic B, Stranick M, Somasundaran P. Adsorption and dissolution behavior of human plasma fibronectin on thermally and chemically modified titanium dioxide particles. Biomaterials. 2002; 23:1269–79.

40. Rupp F, Scheideler L, Rehbein D, Axmann D, Geis-Gerstorfer J. Roughness induced dynamic changes of wettability of acid etched titanium implant modifications. Biomaterials. 2004; 25:1429–38.

Go to : 
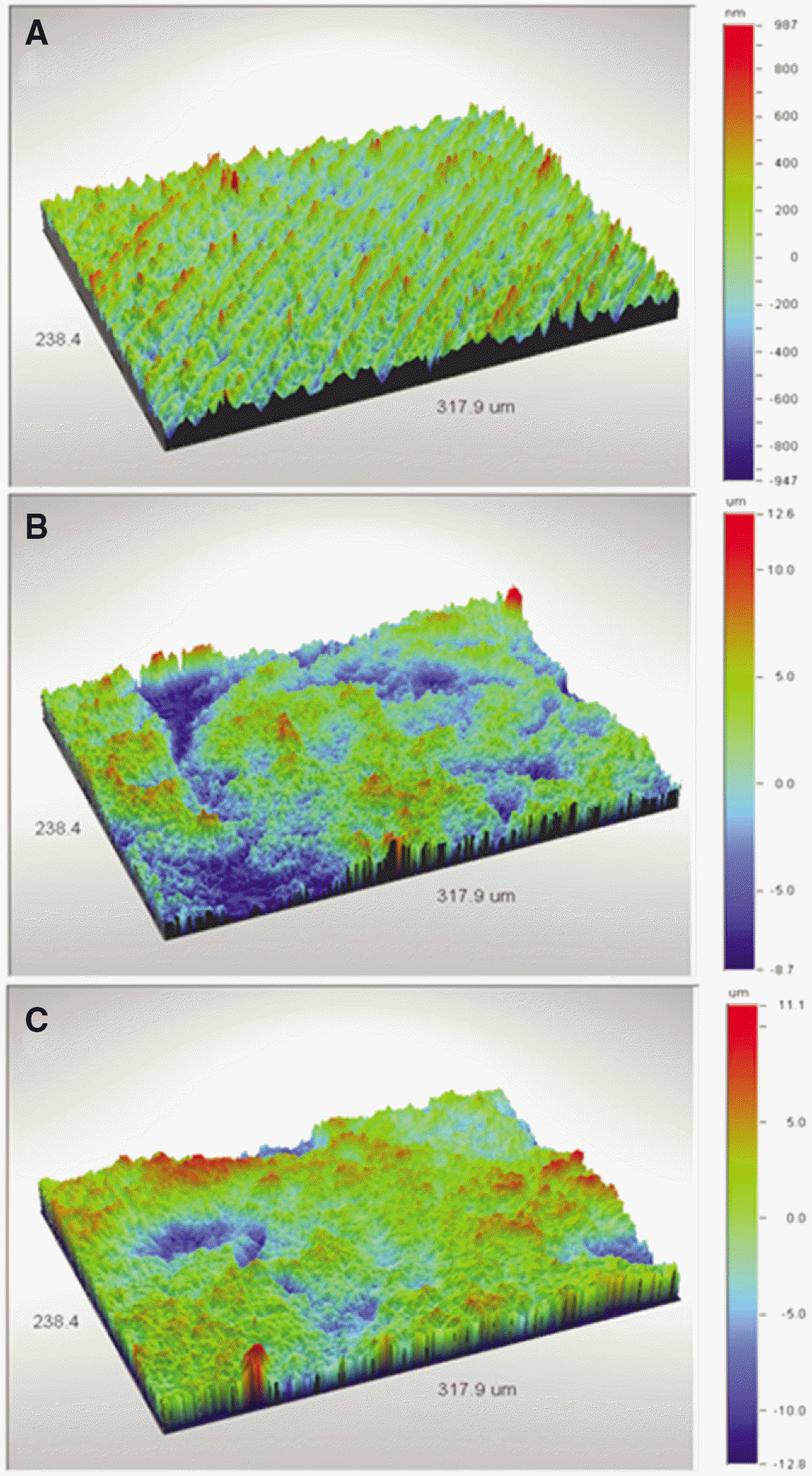 | Fig. 1.Non-Contact 3D Video Measuring images of each group. (A) Machined,(B) SLA, (C) SLA/NaOH group. |
 | Fig. 2.scanning electron microscope (Hitachi S-4700 SEM, Japan) images of each group (×2000 magnification). (A) Machined, (B) SLA, (C) SLA/NaOH group. |
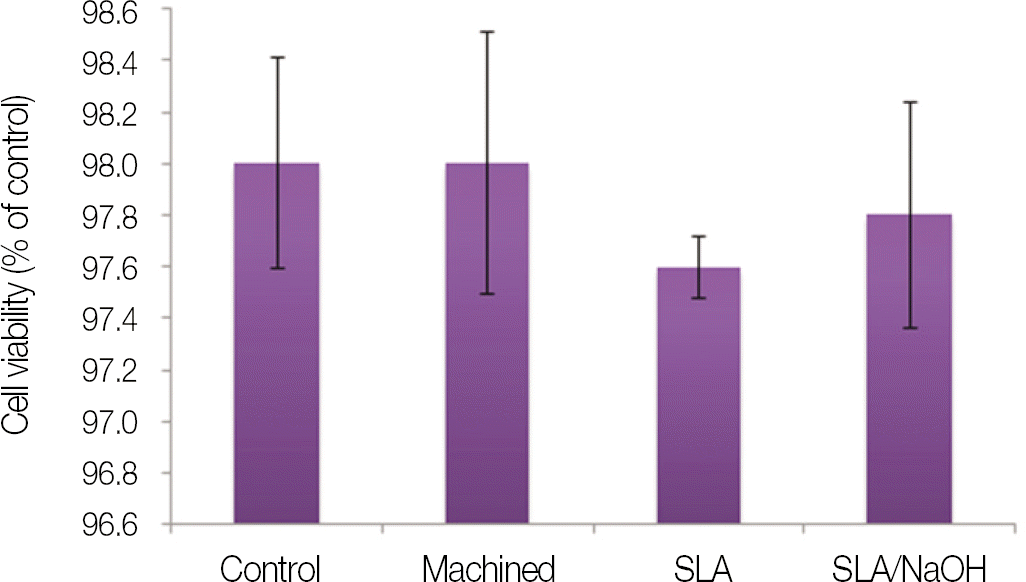 | Fig. 4.Cytotoxicity test of machined group, SLA group and SLA/NaOH group against osteoblasts (MG-63 cells). |
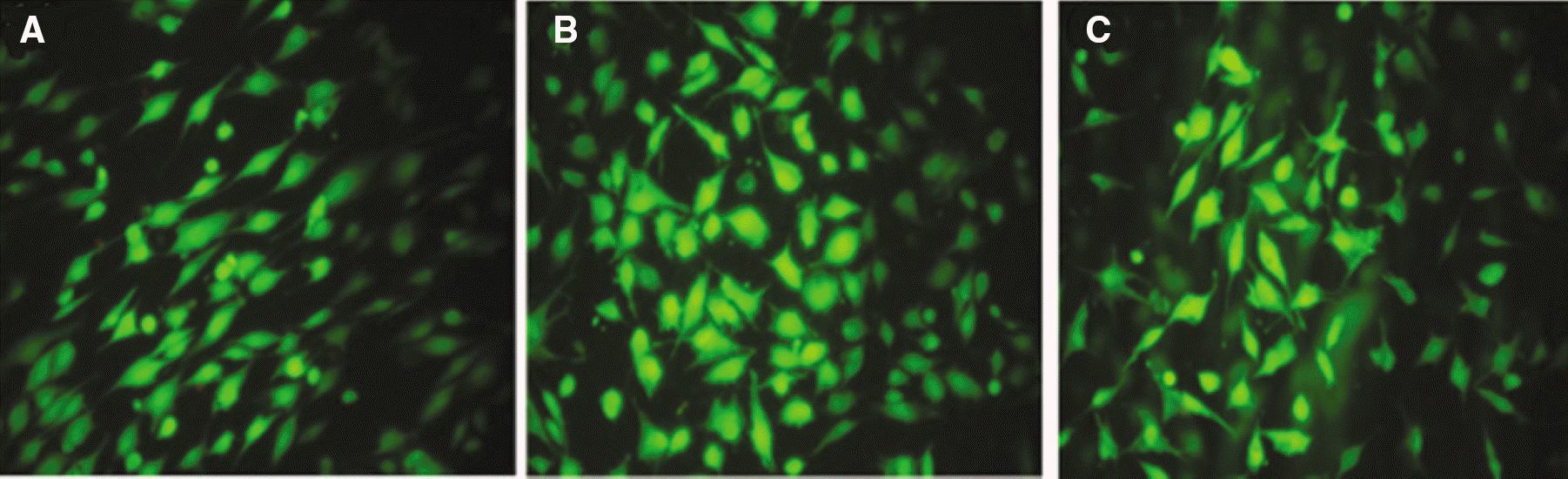 | Fig. 5.Live/dead assay for machined group, SLA group and SLA/NaOH group against osteoblasts (MG-63 cells). |
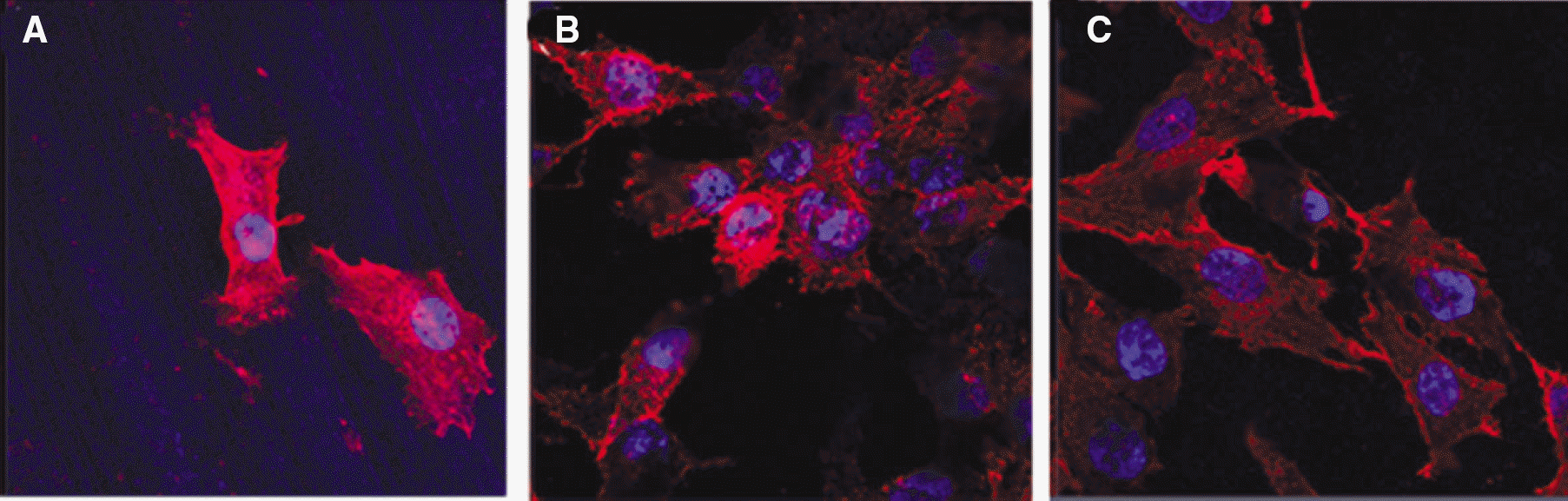 | Fig. 6.Cells adhesion and spread of MG-63 cells on machined group, SLA group and SLA/NaOH group after 1 day. |
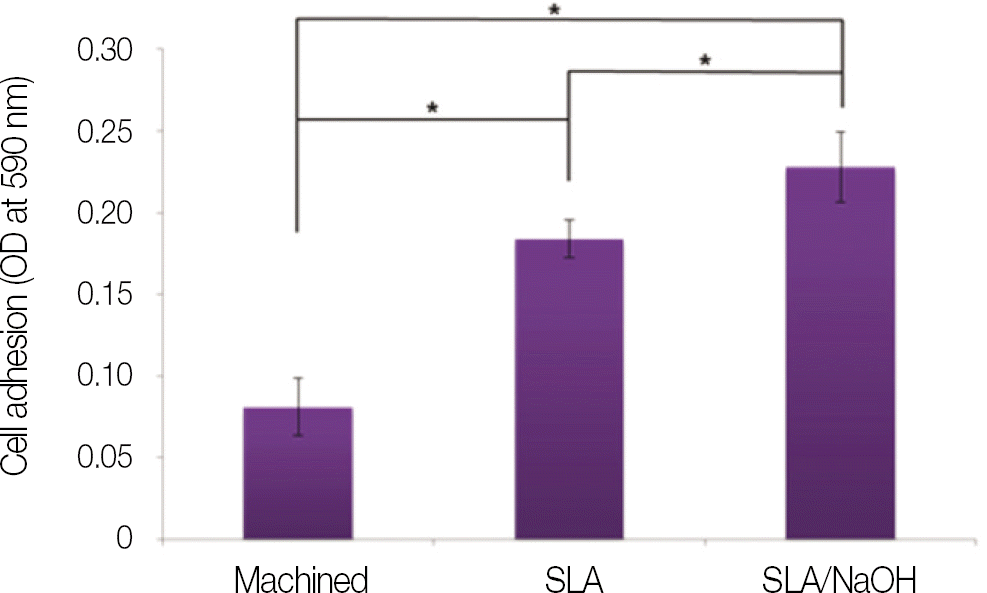 | Fig. 7.Cells adhesion of MG-63 cells on machined group, SLA group and SLA/NaOH group after 3 h. |
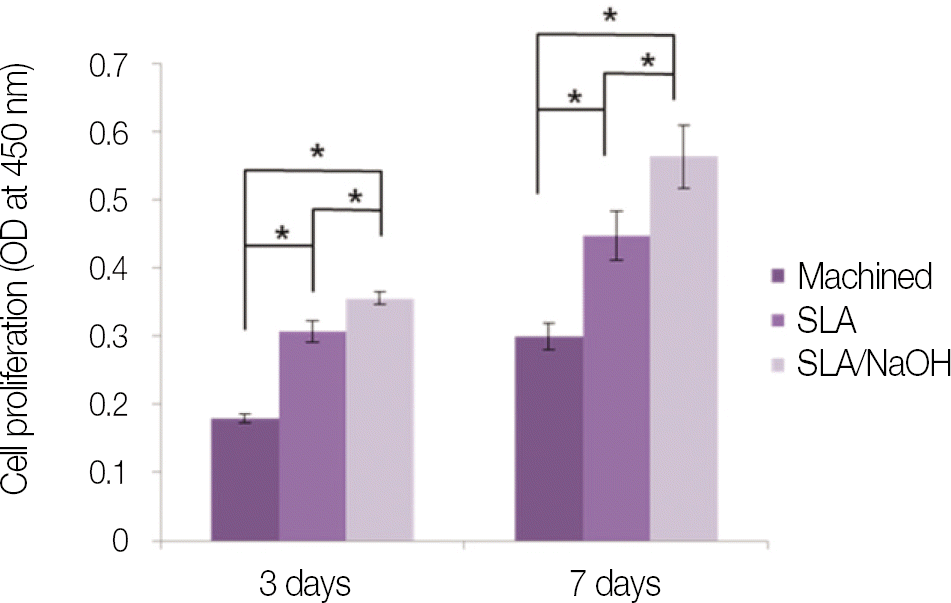 | Fig. 8.Cell proliferation of osteoblast-like cells (MG-63 cells) grown on machined group, SLA group and SLA/NaOH group after day 3, and day 7. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download