Abstract
Purpose
The purposes of this study were to investigate osseointegration around zirconia implants which had machined or alumina sandblasted surface, and to compare the results with titanium implants.
Materials and methods
The study was performed on the tibia of 6 pigs. Three types of implants were investigated: group T-titanium implant, group Z-machined zirconia implant, group ZS-alumina sandblasting treated zirconia implant. Zirconia implants were manufactured from yttria-stabilized tetragonal zirconia polycrystalline (Acucera Inc., Pocheon, Korea). A total of 36 implants were installed in pigs' tibias. After 1, 4 and 12 weeks of healing period, the periotest and the histomorphometric analysis were performed. The data were analyzed using one-way ANOVA and significance was assessed by the Scheffe test (α =.05).
Results
In the measurement of surface roughness, highest Ra value was measured in group T with significant difference. No significant differences were found among groups regarding Periotest values. After 1 week, in comparison of bone to implant contact (BIC), group Z showed higher value with significant difference. In comparison of bone area (BA), group T and group Z showed higher value with significant difference than group ZS. After 4 weeks, in comparison of BIC, group T showed higher value with significant difference. Comparison of BA showed no significant difference among each implant. After 12 weeks, the highest mean BIC values were found in group T with significant difference. Group ZS showed higher BIC value with significant difference than group Z. In comparison of BA, group T and group ZS showed higher value with significant difference than group Z.
Go to : 
REFERENCES
1.Stadlinger B., Hennig M., Eckelt U., Kuhlisch E., Mai R. Comparison of zirconia and titanium implants after a short healing period. A pilot study in minipigs. Int J Oral Maxillofac Surg. 2010. 39:585–92.

2.Heydecke G., Kohal R., Gla¨ser R. Optimal esthetics in single-tooth replacement with the Re-Implant system: a case report. Int J Prosthodont. 1999. 12:184–9.
3.Stejskal J., Stejskal VD. The role of metals in autoimmunity and the link to neuroendocrinology. Neuro Endocrinol Lett. 1999. 20:351–64.
4.Weingart D., Steinemann S., Schilli W., Strub JR., Hellerich U., Assenmacher J., Simpson J. Titanium deposition in regional lymph nodes after insertion of titanium screw implants in maxillofacial region. Int J Oral Maxillofac Surg. 1994. 23:450–2.

5.Akagawa Y., Ichikawa Y., Nikai H., Tsuru H. Interface histology of unloaded and early loaded partially stabilized zirconia endosseous implant in initial bone healing. J Prosthet Dent. 1993. 69:599–604.

6.Hoffmann O., Angelov N., Gallez F., Jung RE., Weber FE. The zirconia implant-bone interface: a preliminary histologic evaluation in rabbits. Int J Oral Maxillofac Implants. 2008. 23:691–5.
7.Andreiotelli M., Kohal RJ. Fracture strength of zirconia implants after artificial aging. Clin Implant Dent Relat Res. 2009. 11:158–66.

8.Ichikawa Y., Akagawa Y., Nikai H., Tsuru H. Tissue compatibility and stability of a new zirconia ceramic in vivo. J Prosthet Dent. 1992. 68:322–6.

9.Kohal RJ., Weng D., Ba¨chle M., Strub JR. Loaded custom-made zirconia and titanium implants show similar osseointegration: an animal experiment. J Periodontol. 2004. 75:1262–8.

10.Nebe B., Forster C., Pommerenke H., Fulda G., Behrend D., Bernewski U., Schmitz KP., Rychly J. Structural alterations of adhesion mediating components in cells cultured on poly-beta-hydroxy butyric acid. Biomaterials. 2001. 22:2425–34.
11.Anselme K., Bigerelle M., Noel B., Dufresne E., Judas D., Iost A., Hardouin P. Qualitative and quantitative study of human osteoblast adhesion on materials with various surface roughnesses. J Biomed Mater Res. 2000. 49:155–66.

12.Lincks J., Boyan BD., Blanchard CR., Lohmann CH., Liu Y., Cochran DL., Dean DD., Schwartz Z. Response of MG63 osteoblast-like cells to titanium and titanium alloy is dependent on surface roughness and composition. Biomaterials. 1998. 19:2219–32.

13.Lee JH., Lim HS., Lim JH., Cho IH. The effect of resorbable membrane and xenogenic graft materials on implant stability and periimplant tissue reaction. J Korean Acad Oral Maxillofac Implants. 2001. 5:70–95.
14.Yim JH., Lim HS., Lim JH., Cho IH. The effect of various graft materials on the stability of implant and periimplant tissue response in rabbit tibia. J Korean Acad Oral Maxillofac Implants. 2001. 5:41–64.
15.Wennerberg A., Albrektsson T. Suggested guidelines for the topographic evaluation of implant surfaces. Int J Oral Maxillofac Implants. 2000. 15:331–44.
16.Albrektsson T., Wennerberg A. Oral implant surfaces: Part 1-review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int J Prosthodont. 2004. 17:536–43.
17.Kieswetter K., Schwartz Z., Dean DD., Boyan BD. The role of implant surface characteristics in the healing of bone. Crit Rev Oral Biol Med. 1996. 7:329–45.

18.Hempel U., Hefti T., Kalbacova M., Wolf-Brandstetter C., Dieter P., Schlottig F. Response of osteoblast-like SAOS-2 cells to zirconia ceramics with different surface topographies. Clin Oral Implants Res. 2010. 21:174–81.

19.Palmieri A., Pezzetti F., Brunelli G., Lo Muzio L., Scarano A., Scapoli L., Martinelli M., Arlotti M., Guerzoni L., Rubini C., Carinci F. Short-period effects of zirconia and titanium on osteoblast microRNAs. Clin Implant Dent Relat Res. 2008. 10:200–5.

20.Schliephake H., Scharnweber D. Chemical and biological functionalization of titanium for dental implants. J Mater Chem. 2008. 18:2404–14.

21.Roessler S., Zimmermann R., Scharnweber D., Werner C., Worch H. Characterization of oxide layers on Ti6Al4V and titanium by streaming potential and streaming current measurements. Colloid Surf B: Biointerfaces. 2002. 26:387–95.

22.Leong YK., Scales PJ., Healy TW., Boger DV. Effect of particle size on colloidal zirconia rheology at the isoelectric point. J Am Ceram Soc. 1995. 78:2209–12.

23.Shin D., Blanchard SB., Ito M., Chu TM. Peripheral quantitative computer tomographic, histomorphometric, and removal torque analyses of two different non-coated implants in a rabbit model. Clin Oral Implants Res. 2011. 22:242–50.

24.Schliephake H., Hefti T., Schlottig F., Ge′det P., Staedt H. Mechanical anchorage and periimplant bone formation of surface-modified zirconia in minipigs. J Clin Periodontol. 2010. 37:818–28.

25.Langhoff JD., Voelter K., Scharnweber D., Schnabelrauch M., Schlottig F., Hefti T., Kalchofner K., Nuss K., von Rechenberg B. Comparison of chemically and pharmaceutically modified titanium and zirconia implant surfaces in dentistry: a study in sheep. Int J Oral Maxillofac Surg. 2008. 37:1125–32.

Go to : 
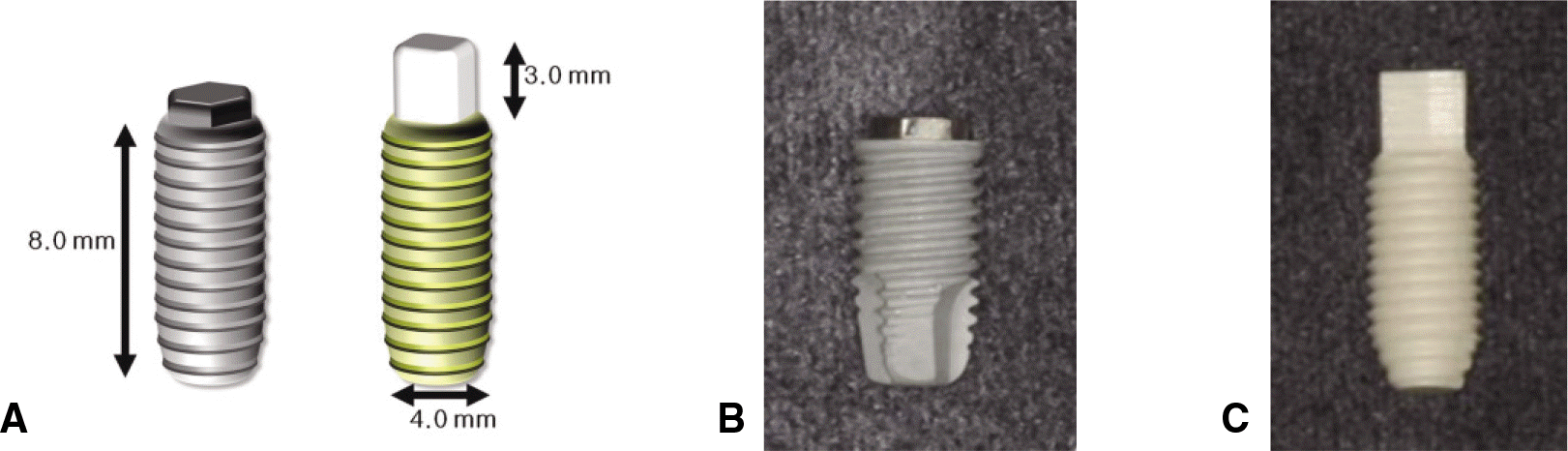 | Fig. 1.Implants used in this study. A: Mimetic diagram of implants, B: Titanium implant, C: Zirconia implant. |
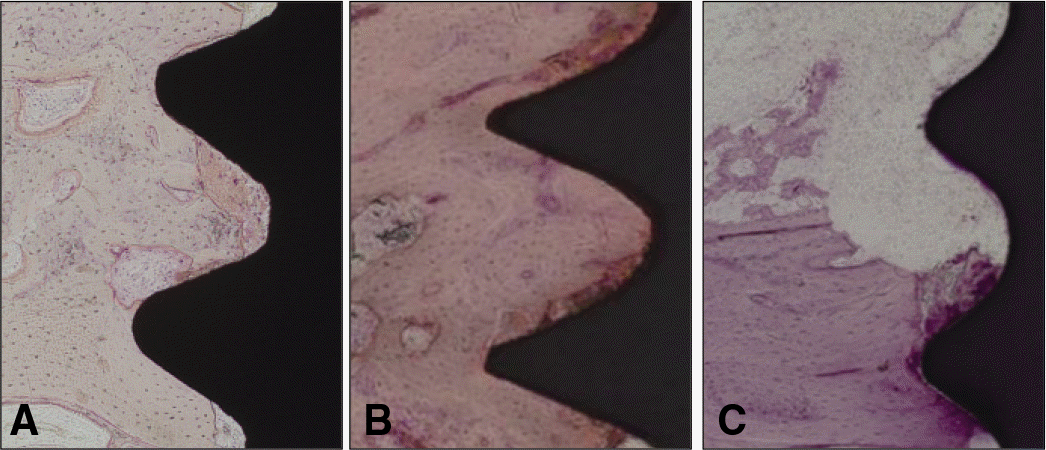 | Fig. 2.Light micrographs taken 1 week after insertion. A: Group T, B: Group Z, C: Group ZS, The thread area was occupied by old bone, bone fragments and red blood cells. Inflammatory cell can be detected (H-E, magnification ×100). |
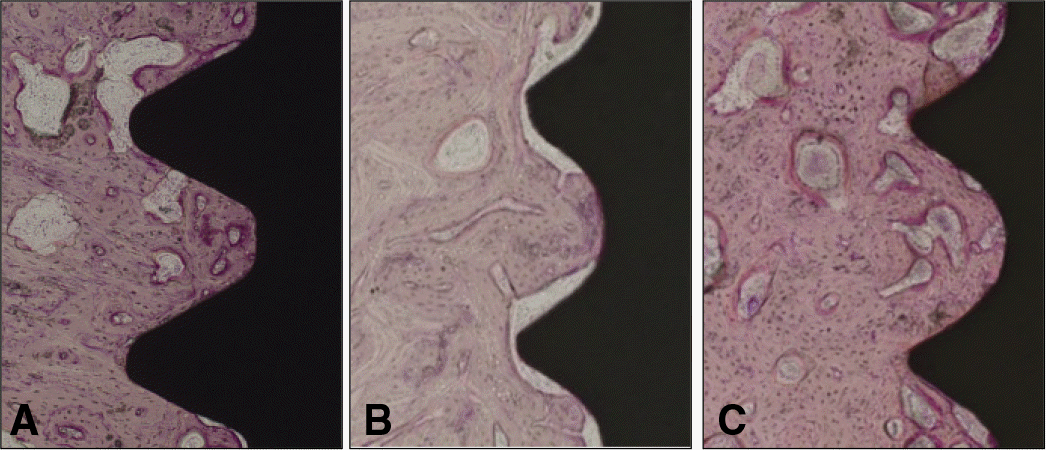 | Fig. 3.Light micrographs taken 4 weeks after insertion. A: Group T, B: Group Z, C: Group ZS, The thread was occupied by new bone. Fibrous/vascular tissue between bone and machined zirconia implant were observed (H-E, ×100). |
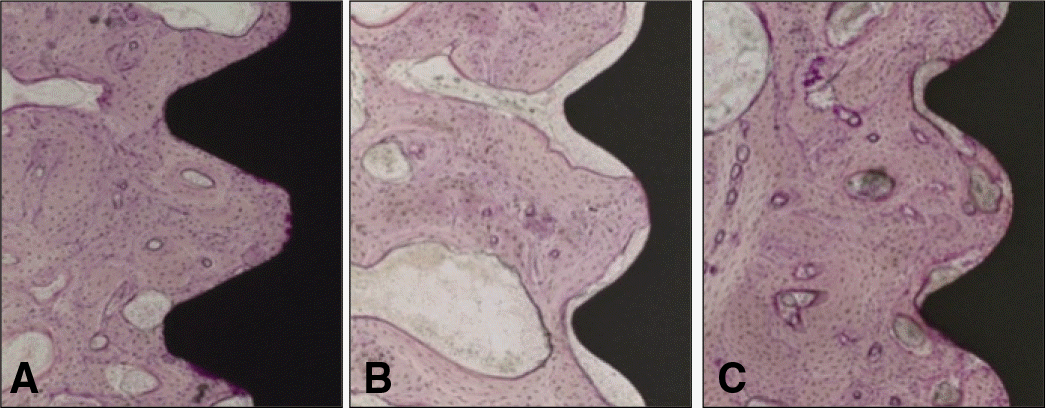 | Fig. 4.Light micrographs taken 12 weeks after insertion. A: Group T, Bone formation with high bone contact adjacent to titanium implants was seen. B: Group Z, A fibrous capsule was present in the interface. C: Group ZS, Thin fibrous capsule between bone and sandblasting treated zirconia implant was seen (H-E, ×100). |
 | Fig. 5.Scanning electron micrographs of bone to implant contact taken 1 week after insertion. A: Group T, B: Group Z, C: Group ZS, Bone formation was evident near the implant surface (×1,500). |
 | Fig. 6.Scanning electron micrographs of bone to implant contact taken 4 weeks after insertion. A: Group T, An unmineralized zone separated the mineralized bone from the implant surface (×1,500). B: Group Z, Interface tissue between calcified bone and implant (×1,500). C: Group ZS, A dense amorphous layer was located at the implant surface (×1,500). |
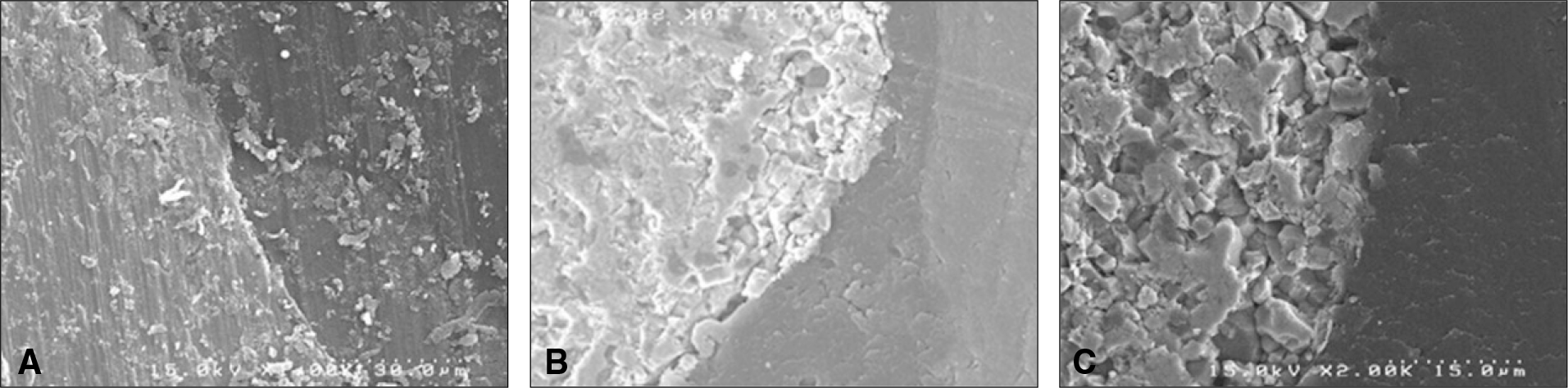 | Fig. 7.Scanning electron micrographs of bone to implant contact taken 12 weeks after insertion. A: Group T, An intimate contact can be detected between the bone and titanium implant (×1,500). B: Group Z, A part of newly formed tissue was detached from the zirconia implant (×1,500). C: Group ZS, A direct bone contact was observed (×1,500). |
Table 1.
Classification of control and experimental groups in this study
| Group | Material | Surface preparation | Weeks | N |
|---|---|---|---|---|
| T (control) | Titanium (grade IV) | Anodic oxidation | 1 | 4 |
| 4 | 4 | |||
| 12 | 4 | |||
| Z (experimental) | 3Y-TZP∗ | Machined | 1 | 4 |
| 4 | 4 | |||
| 12 | 4 | |||
| ZS (experimental) | 3Y-TZP∗ | Al2O3 sandblasted | 1 | 4 |
| 4 | 4 | |||
| 12 | 4 |
Table 2.
Distribution analysis of surface roughness
| Group | Mean | SD | P value | Scheffe PHT |
|---|---|---|---|---|
| T | 0.70 | 0.03 | ||
| Z | 0.26 | 0.01 | 0.00∗ | (Z=ZS) < T |
| ZS | 0.29 | 0.03 |
Table 3.
Mean and standard deviation of PTVs
| Group | 0 week | 1 week | 4 weeks | 12 weeks |
|---|---|---|---|---|
| T | -4.6 ± 2.6 | 2.3 ± 5.0 | -6.0 ± 2.2 | -6.0 ± 0.0 |
| Z | -1.5 ± 3.2 | 0.0 ± 3.4 | -2.8 ± 7.2 | -4.0 ± 2.4 |
| ZS | -1.6 ± 5.8 | -3.0 ± 6.0 | -1.0 ± 7.9 | -4.8 ± 2.1 |
Table 4.
Measured percentage of bone to implant contact (mean ± SD)
| Group | Bone to implant contact (%) | ||
|---|---|---|---|
| 1 week | 4 weeks | 12 weeks | |
| T | 26.8 ± 2.8 | 70.3 ± 8.9† | 84.8 ± 2.9‡ |
| Z | 53.8 ± 24.1∗ | 27.8 ± 11.1 | 26.0 ± 13.7‡ |
| ZS | 10.5 ± 7.9 | 36.0 ± 21.6 | 43.0 ± 7.4‡ |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download