Abstract
Purpose
The purpose of this study was to investigate the effect of different administration duration of alendronate on initial wound healing and new bone formation of extraction socket in rats.
Materials and methods
Fifteen male Sprague-Dawley rats (body weight 130-140 g, 4 weeks old, male) were divided into control group (no alendronate administration) and experimental group (alendronate administration). Experimental group was subdivided into 1 week administrated group, 2 week administrated group, 4 week administrated group and 6 week administrated group according to duration of administration. For the experimental groups, during the designated time period (at the time of extraction, 1 week before extraction, 3 week before extraction and 5 week before extraction) till 1 week after extraction, rats were subcutaneously injected with Alendronate at the dose of 1.0 mg/Kg three times a week. Each specimen from 6 week experimental group and control group were used for microarray analysis, and other specimens were used for histological analysis. The rate of new bone formation within the extraction site and bone loss activity was analyzed using TRAP staining. Statistical analysis was performed using Kruskal Wallis test. (α =.05)
Results
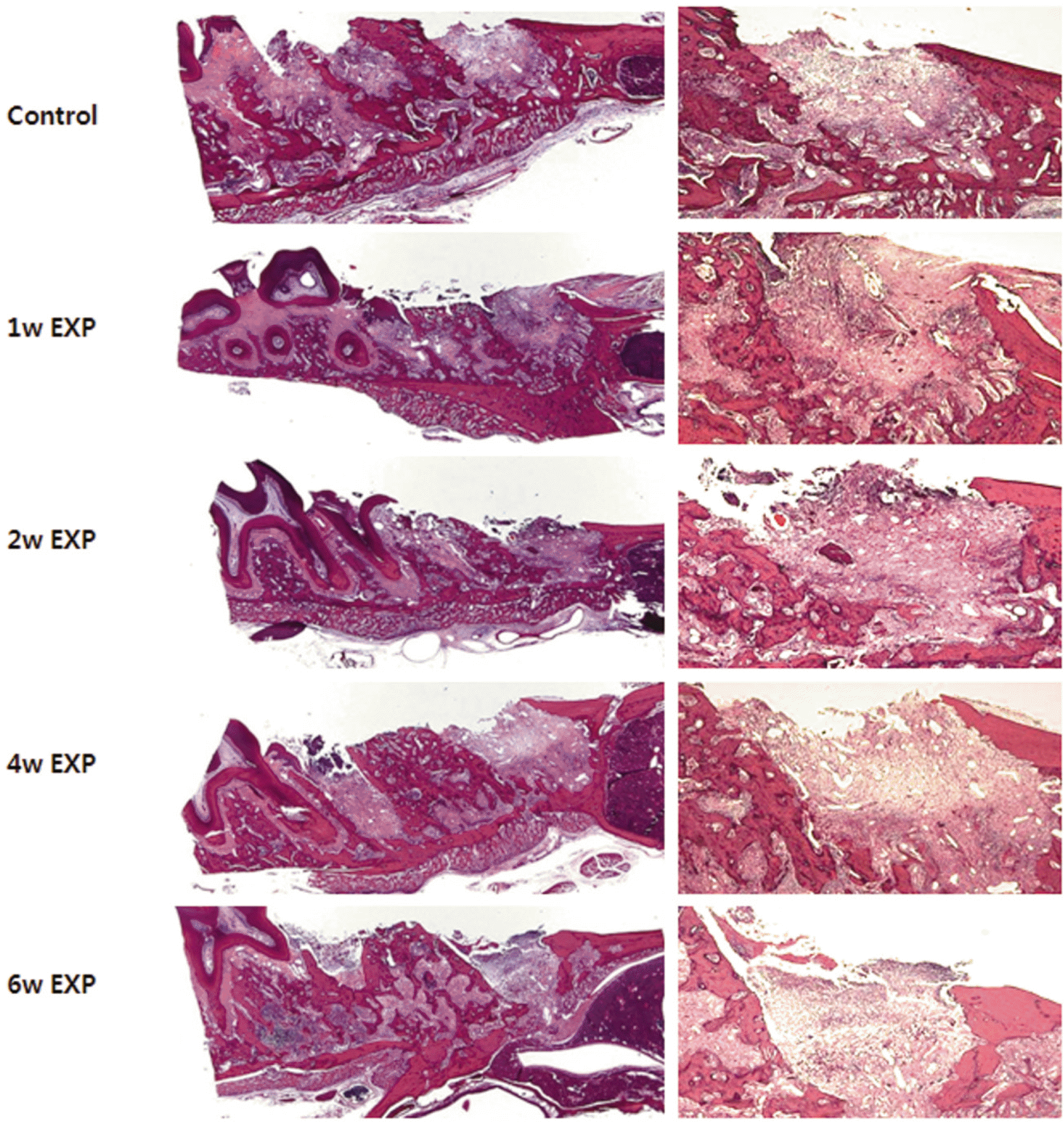
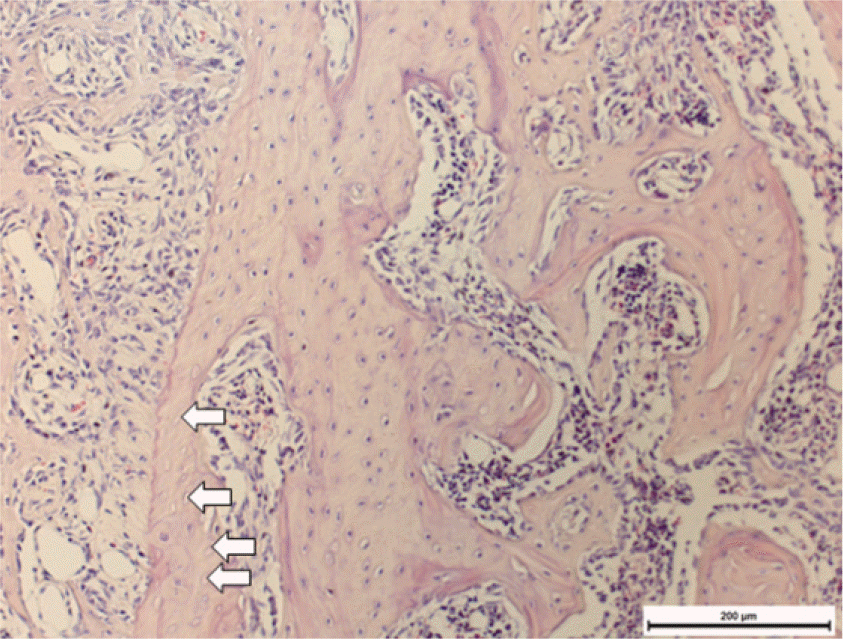
After one week from the time of extraction, the rate of new bone formation within extraction site for the control group (16.77% ± 1.36%) compared to the 4 week experimental group (14.99% ± 6.26%) was lower. However, no statistically significant difference was found. Increase in the number of inactive lacuna (empty lacuna) and decrease in the number of TRAP positive cell were identified with increased duration of administration. There was no significant difference.
Go to : 
REFERENCES
1.Wood J., Bonjean K., Ruetz S., Bellahce`ne A., Devy L., Foidart JM., Castronovo V., Green JR. Novel antiangiogenic effects of the bisphosphonate compound zoledronic acid. J Pharmacol Exp Ther. 2002. 302:1055–61.

2.Fournier P., Boissier S., Filleur S., Guglielmi J., Cabon F., Colombel M., Cle′zardin P. Bisphosphonates inhibit angiogenesis in vitro and testosterone-stimulated vascular regrowth in the ventral prostate in castrated rats. Cancer Res. 2002. 62:6538–44.
3.Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003. 61:1115–7.

4.Ruggiero SL., Mehrotra B., Rosenberg TJ., Engroff SL. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg. 2004. 62:527–34.

5.Lazarovici TS., Yahalom R., Taicher S., Schwartz-Arad D., Peleg O., Yarom N. Bisphosphonate-related osteonecrosis of the jaw associated with dental implants. J Oral Maxillofac Surg. 2010. 68:790–6.

6.Marx RE., Sawatari Y., Fortin M., Broumand V. Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg. 2005. 63:1567–75.

7.Estilo CL., Van Poznak CH., Wiliams T., Bohle GC., Lwin PT., Zhou Q., Riedel ER., Carlson DL., Schoder H., Farooki A., Fornier M., Halpern JL., Tunick SJ., Huryn JM. Osteonecrosis of the maxilla and mandible in patients with advanced cancer treated with bisphosphonate therapy. Oncologist. 2008. 13:911–20.

8.Allen MR., Burr DB. The pathogenesis of bisphosphonate-related osteonecrosis of the jaw: so many hypotheses, so few data. J Oral Maxillofac Surg. 2009. 67:61–70.

9.Hikita H., Miyazawa K., Tabuchi M., Kimura M., Goto S. Bisphosphonate administration prior to tooth extraction delays initial healing of the extraction socket in rats. J Bone Miner Metab. 2009. 27:663–72.

10.Kobayashi Y., Hiraga T., Ueda A., Wang L., Matsumoto-Nakano M., Hata K., Yatani H., Yoneda T. Zoledronic acid delays wound healing of the tooth extraction socket, inhibits oral epithelial cell migration, and promotes proliferation and adhesion to hydroxyapatite of oral bacteria, without causing osteonecrosis of the jaw, in mice. J Bone Miner Metab. 2010. 28:165–75.

11.Yamamoto Y., Udagawa N., Matsuura S., Nakamichi Y., Horiuchi H., Hosoya A., Nakamura M., Ozawa H., Takaoka K., Penninger JM., Noguchi T., Takahashi N. Osteoblasts provide a suitable mi-croenvironment for the action of receptor activator of nuclear factor-kappaB ligand. Endocrinology. 2006. 147:3366–74.
12.Tabuchi M., Miyazawa K., Kimura M., Maeda H., Kawai T., Kameyama Y., Goto S. Enhancement of crude bone morphogenetic protein-induced new bone formation and normalization of endochondral ossification by bisphosphonate treatment in osteo-protegerin-deficient mice. Calcif Tissue Int. 2005. 77:239–49.

13.Graziani F., Rosini S., Cei S., La Ferla F., Gabriele M. The effects of systemic alendronate with or without intraalveolar collagen sponges on postextractive bone resorption: a single masked randomized clinical trial. J Craniofac Surg. 2008. 19:1061–6.
14.Miettinen SS., Jaatinen J., Pelttari A., Lappalainen R., Mo¨nkko¨nen J., Venesmaa PK., Kro¨ger HP. Effect of locally administered zoledronic acid on injury-induced intramembranous bone regeneration and osseointegration of a titanium implant in rats. J Orthop Sci. 2009. 14:431–6.

15.Viera-Negro′n YE., Ruan WH., Winger JN., Hou X., Sharawy MM., Borke JL. Effect of ovariectomy and alendronate on implant osseointegration in rat maxillary bone. J Oral Implantol. 2008. 34:76–82.
16.Kim JH., Park YB., Li Z., Shim JS., Moon HS., Jung HS., Chung MK. Effect of alendronate on healing of extraction sockets and healing around implants. Oral Dis. 2011. 17:705–11.

17.Aguirre JI., Altman MK., Vanegas SM., Franz SE., Bassit AC., Wronski TJ. Effects of alendronate on bone healing after tooth extraction in rats. Oral Dis. 2010. 16:674–85.

18.Pereira MC., Zecchin KG., Campagnoli EB., Jorge J. Ovariectomy delays alveolar wound healing after molar extractions in rats. J Oral Maxillofac Surg. 2007. 65:2248–53.

19.Chacon GE., Stine EA., Larsen PE., Beck FM., McGlumphy EA. Effect of alendronate on endosseous implant integration: an in vivo study in rabbits. J Oral Maxillofac Surg. 2006. 64:1005–9.

20.Kurth AH., Eberhardt C., Mu¨ller S., Steinacker M., Schwarz M., Bauss F. The bisphosphonate ibandronate improves implant integration in osteopenic ovariectomized rats. Bone. 2005. 37:204–10.

21.Otto S., Abu-Id MH., Fedele S., Warnke PH., Becker ST., Kolk A., Mu¨cke T., Mast G., Ko¨hnke R., Volkmer E., Haasters F., Lieger O., Iizuka T., Porter S., Campisi G., Colella G., Ploder O., Neff A., Wiltfang J., Ehrenfeld M., Kreusch T., Wolff KD., Stu¨rzenbaum SR., Schieker M., Pautke C. Osteoporosis and bisphosphonates-related osteonecrosis of the jaw: not just a sporadic coincidence-a multi-centre study. J Craniomaxillofac Surg. 2011. 39:272–7.
22.Iizuka T., Miller SC., Marks SC Jr. Alveolar bone remodeling after tooth extraction in normal and osteopetrotic (ia) rats. J Oral Pathol Med. 1992. 21:150–5.

23.Kenzora JE., Steele RE., Yosipovitch ZH., Glimcher MJ. Experimental osteonecrosis of the femoral head in adult rabbits. Clin Orthop Relat Res. 1978. 130:8–46.

24.Futami T., Fujii N., Ohnishi H., Taguchi N., Kusakari H., Ohshima H., Maeda T. Tissue response to titanium implants in the rat maxilla: ultrastructural and histochemical observations of the bone-titanium interface. J Periodontol. 2000. 71:287–98.

25.Shimizu M., Furuya R., Kawawa T., Sasaki T. Bone wound healing after maxillary molar extraction in ovariectomized aged rats: quantitative backscattered electron image analysis. Anat Rec. 2000. 259:76–85.

26.Brennan O., Kennedy OD., Lee TC., Rackard SM., O'Brien FJ. Effects of estrogen deficiency and bisphosphonate therapy on osteocyte viability and microdamage accumulation in an ovine model of osteoporosis. J Orthop Res. 2011. 29:419–24.

Go to : 
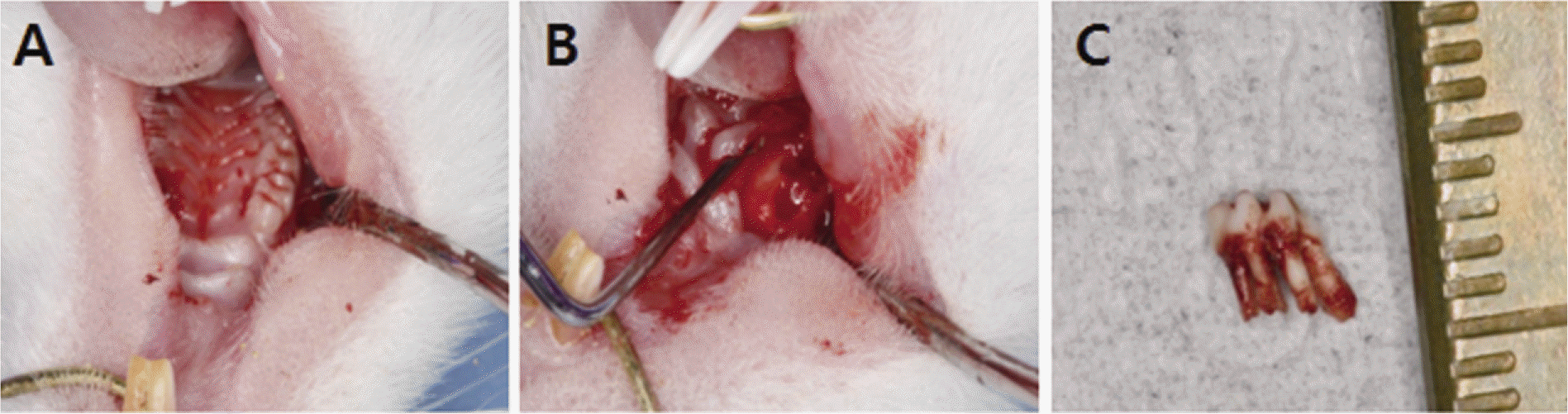 | Fig. 1.Extraction socket of the first molar of the rat. A: Right maxillary first molar of the rat, B: Extraction socket C: Extracted maxillary first molar. |
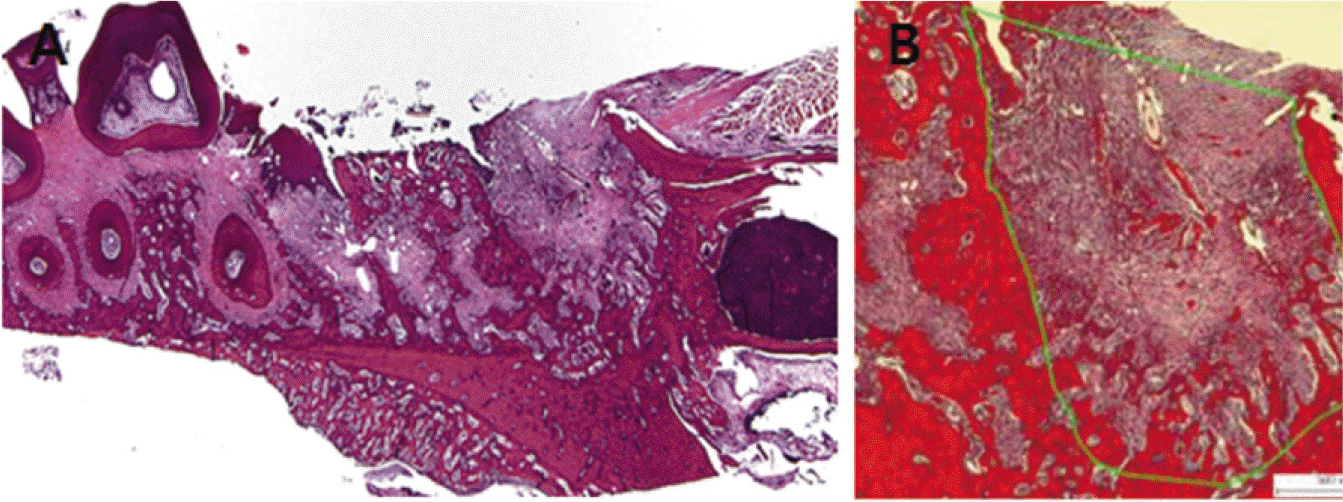 | Fig. 2.A: Interested area of extraction socket (H&E stain ×12.5), B: Border of mesial root (H&E stain ×50) - Image Pro plus program version 4.5 software (Media cybernetics Inc, USA). |
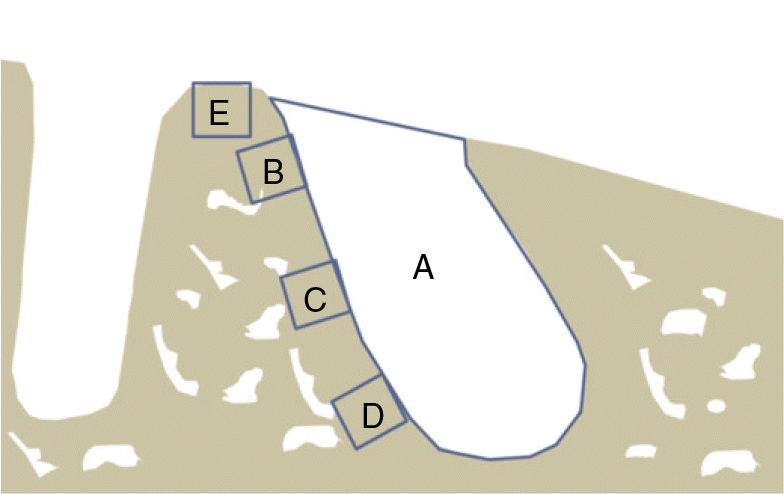 | Fig. 3.The selected area for measurement in the extracted socket of mesial root. A: Extracted socket area of the mesial root of the maxillary first molar, B-D: 3 lateral areas beside the mesial root, E: Septum area. Each blue square (300 ㎛× 300 ㎛). |
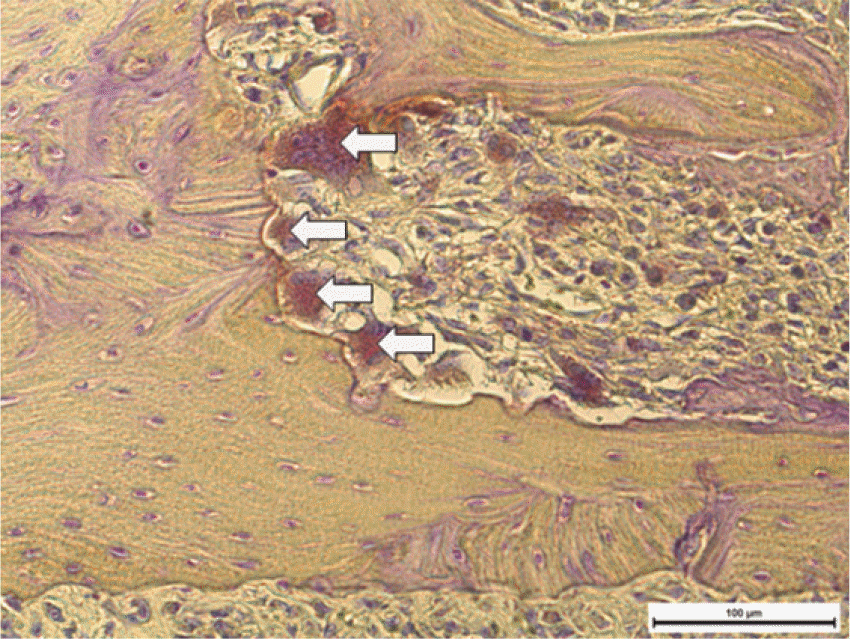 | Fig. 6.TRAP positive cell counting (Right: TRAP, ×200): White arrows indicate TRAP positive cells. |
Table 1.
Study design
Table 2.
Newly formed bone area (%) in mesial root area of extraction socket
| Con | 1 w EXP | 2 w EXP | 4 w EXP | |
|---|---|---|---|---|
| Bone area (%) | 16.77 ± 1.36 | 17.66 ± 2.76 | 16.94 ± 2.84 | 14.99 ± 6.26 |
Table 3.
Empty lacunae (non-viable osteocyte) cell numbers counting in septum area & TRAP positive cell numbers counting in septum area
| Con | 1 w EXP | 2 w EXP | 4 w EXP | |
|---|---|---|---|---|
| Empty lacunae cell number | 67 ± 6.25 | 132 ± 52.26 | 164.33 ± 18.58 | 172.33 ± 57.24 |
| TRAP positive cell number | 51 ± 15.71 | 50.33 ± 11.37 | 40.33 ± 10.69 | 39 ± 6.00 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download