Abstract
Purpose
This study evaluated the bonding strength of direct relining resin to Co-Cr denture base material according to surface treatment and immersion time.
Materials and methods
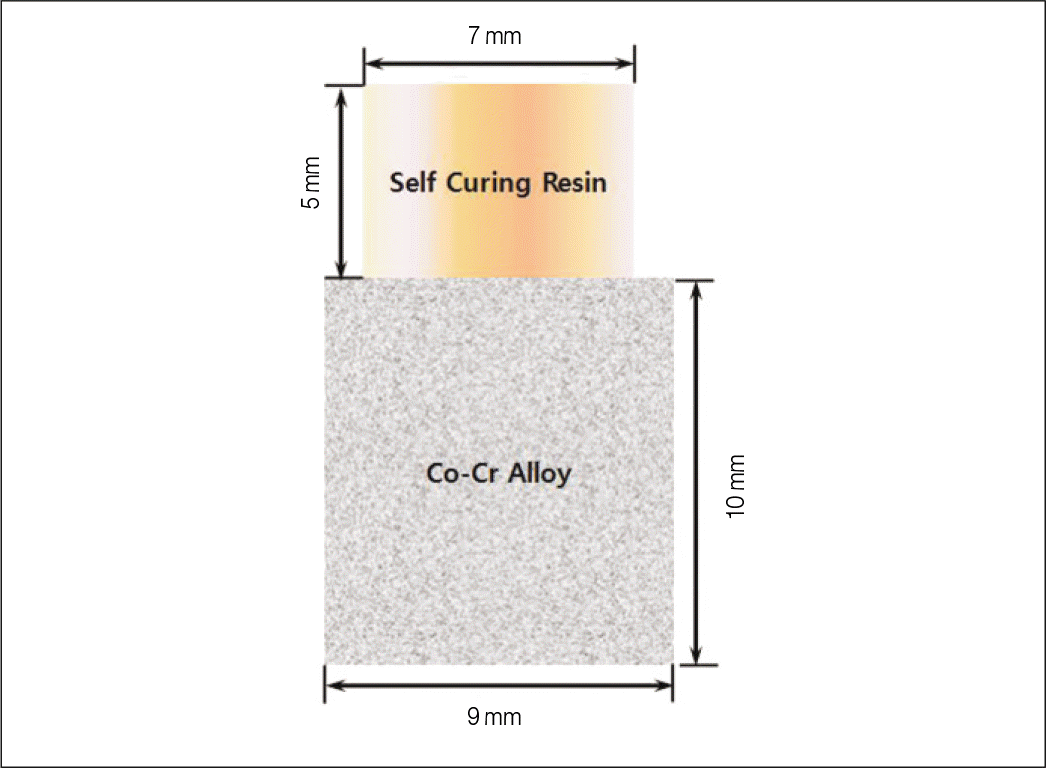
In this study, Co-Cr alloy was used in hexagon shape. Each specimen was cut in flat surface, and sandblasted with 110 μ m Al2O3 for 1 minute. 54 specimens were divided into 3 groups; group A-control group, group B-applied with surface primer A, group C-applied with surface primer B. Self curing direct resin was used for this study. Each group was subdivided into another 3 groups according to the immersion time. After the wetting storage, shear bond strength of the specimens were measured with universal testing machine. The data were analyzed using two-way analysis of variance and Tukey post hoc method.
Results
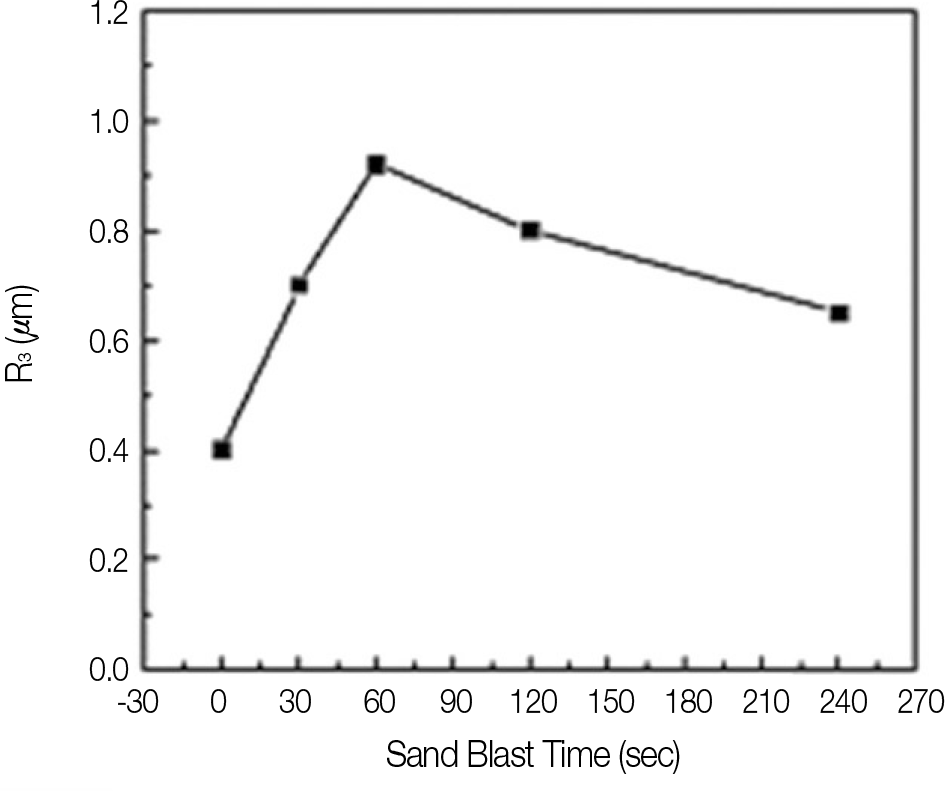
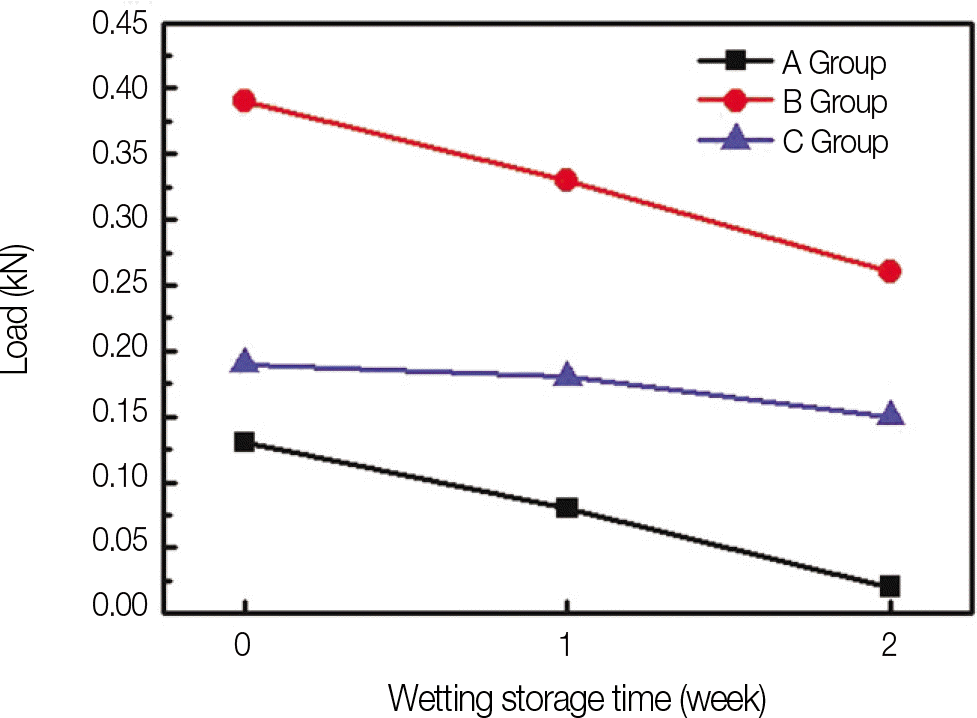
In experiment of sandblasting specimens, surface roughness of the alloy was the highest after 1 minute sandblasting. In experiment of testing shear bond strength, bonding strength was lowered on group B, C, A. There were significant differences between 3 groups. According to period, Bonding strength was the highest on 0 week storage group, and the weakest on 2 week storage group. But there were no significant differences between 3 periods. According to group and period, bonding strength of all group were lowered according to immersion time but there were no significant differences on group B and group C, but there was significant difference according to immersion time on group A.
Go to : 
REFERENCES
1.Kwon KR., Kim YS., Kim CH., Kim HJ., Moon HS., Park SW., Park CJ., Song KY., Lee JS., Lee CH., Lim YJ., Chung MK., Jeong JH., Jeong CM., Cho IH., Cho HW., Choi DG., Han JH. Prosthodontic treatment for edentulous patients. 1st ed.Seoul: Shinhung international;2007. p. 369–75. p. 432–40.
2.McGivney GP., Carr AB. McCracken' s removable partial prosthodontics. 10th ed.St. Louis: CV Mosby;2000. p. 451–5.
3.Brudvik JS. Dental laboratory procedures: complete dentures. St. Louis: CV Mosby;1980. p. 457.
4.Re GJ., Kaiser DA., Malone WF., Garcia-Godoy F. Shear bond strengths and scanning electron microscope evaluation of three different retentive methods for resin-bonded retainers. J Prosthet Dent. 1988. 59:568–73.

5.Krueger GE., Diaz-Arnold AM., Aquilino SA., Scandrett FR. A comparison of electrolytic and chemical etch systems on the resin-to-metal tensile bond strength. J Prosthet Dent. 1990. 64:610–7.

6.Livaditis GJ. A chemical etching system for creating micromechanical retention in resin-bonded retainers. J Prosthet Dent. 1986. 56:181–8.
7.Doukoudakis A., Cohen B., Tsoutsos A. A new chemical method for etching metal frameworks of the acid-etched prosthesis. J Prosthet Dent. 1987. 58:421–3.

8.Sedberry D., Burgess J., Schwartz R. Tensile bond strengths of three chemical and one electrolytic etching systems for a base metal alloy. J Prosthet Dent. 1992. 68:606–10.

9.Zurasky JE., Duke ES. Improved adhesion of denture acrylic resins to base metal alloys. J Prosthet Dent. 1987. 57:520–4.

10.Garfield RE. An effective method for relining metal-based prostheses with acid-etch techniques. J Prosthet Dent. 1984. 51:719–21.

11.Tanaka T., Atsuta M., Nakabayashi N., Masuhara E. Surface treatment of gold alloys for adhesion. J Prosthet Dent. 1988. 60:271–9.

12.Naegeli DG., Duke ES., Schwartz R., Norling BK. Adhesive bonding of composites to a casting alloy. J Prosthet Dent. 1988. 60:279–83.

13.Lyzak WA., Chang JC. Relining a cast palatal denture with a 4-META resin bonding system. Gen Dent. 1995. 43:46–8.
14.Chang JC., Connelly ME., Barclay SM. Relining dentures with metal bases. Am J Dent. 1993. 6:53–4.
15.Park HJ., Kim CW., Kim YS. A study on the flexural bond strength of the gold and the Co-Cr alloy to the denture base resins. J Korean Acad Prosthodont. 2000. 38:500–9.
16.Caeg C., Leinfelder KF., Lacefield WR., Bell W. Effectiveness of a method used in bonding resins to metal. J Prosthet Dent. 1990. 64:37–41.

17.Jacobson TE., Chang JC., Keri PP., Watanabe LG. Bond strength of 4-META acrylic resin denture base to cobalt chromium alloy. J Prosthet Dent. 1988. 60:570–6.

18.Barzilay I., Myers ML., Cooper LB., Graser GN. Mechanical and chemical retention of laboratory cured composite to metal surfaces. J Prosthet Dent. 1988. 59:131–7.

19.Eom TW., Chang IT. The effects of metal surface treatments on the bone strength of polymethyl methacrylate bonded removable prosthese. J Korean Acad Prosthodont. 1998. 36:336–54.
20.May KB., Russell MM., Razzoog ME., Lang BR. The shear strength of polymethyl methacrylate bonded to titanium partial denture framework material. J Prosthet Dent. 1993. 70:410–3.

21.Kolodney H., Puckett AD., Brown K. Shear strength of laboratory-processed composite resins bonded to a silane-coated nickel-chromium-beryllium alloy. J Prosthet Dent. 1992. 67:419–22.

22.van Dalen A., Feilzer AJ., Kleverlaan CJ. The influence of surface treatment and luting cement on in vitro behavior of two-unit cantilever resin-bonded bridges. Dent Mater. 2005. 21:625–32.

23.Tanaka T., Fujiyama E., Shimizu H., Takaki A., Atsuta M. Surface treatment of nonprecious alloys for adhesion-fixed partial dentures. J Prosthet Dent. 1986. 55:456–62.

24.NaBadalung DP., Powers JM., Connelly ME. Comparison of bond strengths of denture base resins to nickel-chromium-beryllium removable partial denture alloy. J Prosthet Dent. 1997. 78:566–73.

25.Matsumura H., Tanaka T., Taira Y., Atsuta M. Bonding of a cobalt-chromium alloy with acidic primers and tri-n-butylborane-initiated luting agents. J Prosthet Dent. 1996. 76:194–9.

26.NaBadalung DP., Powers JM., Connelly ME. Comparison of bond strengths of three denture base resins to treated nickel-chromium-beryllium alloy. J Prosthet Dent. 1998. 80:354–61.

27.Lee JS., Lim JH., Cho IH. A study on the tensile strength between metal denture base and relining materials. J Korean Acad Prosthodont. 2000. 38:1–11.
28.Lee SH., Hwang SH., Moon HS., Lee KW., Shim JS. Shear bond strength of heat-cured denture base resin to surface treated Co-Cr alloy with different methods. J Korean Acad Prosthodont. 2007. 45:216–27.
29.Yoshida K., Taira Y., Matsumura H., Atsuta M. Effect of adhesive metal primers on bonding a prosthetic composite resin to metals. J Prosthet Dent. 1993. 69:357–62.
Go to : 
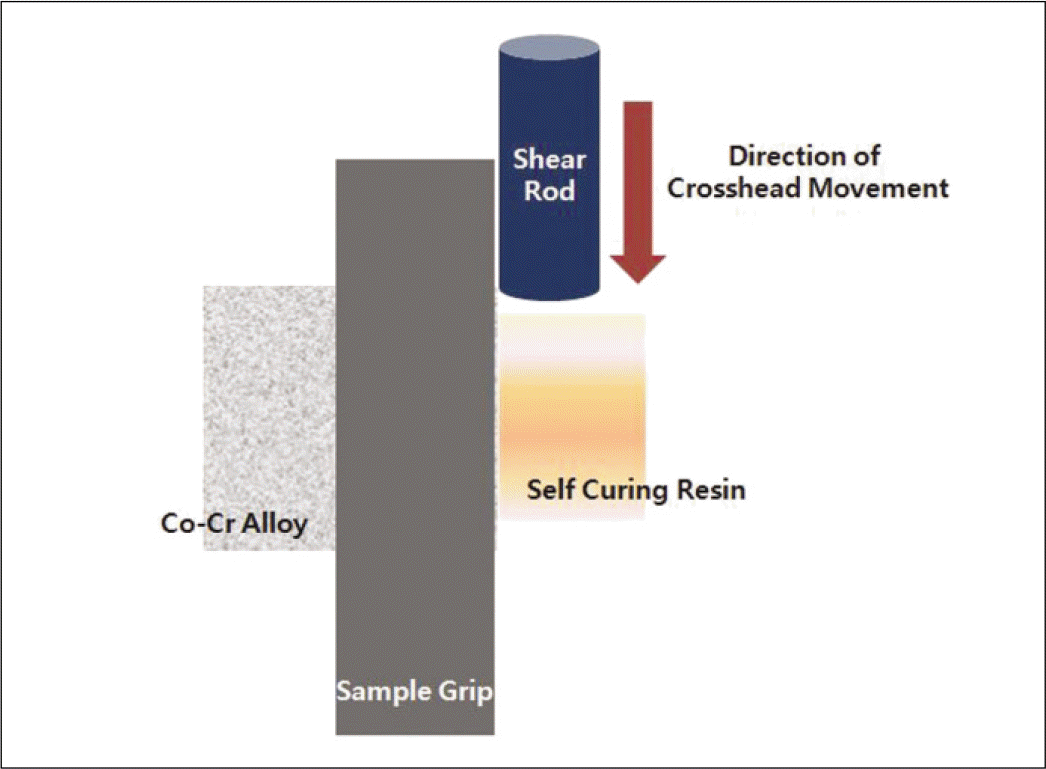
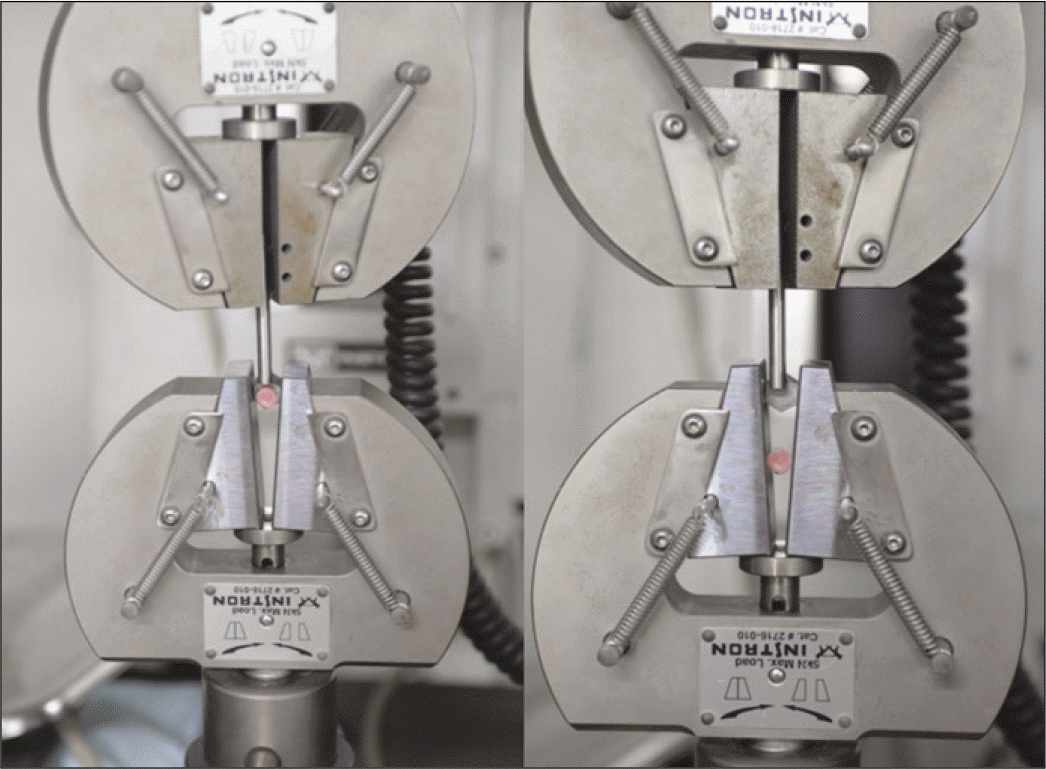 | Fig. 5.Testing shear bond strength (Instron, Universal Testing Machine Model 8872, High Wycombe, UK). |
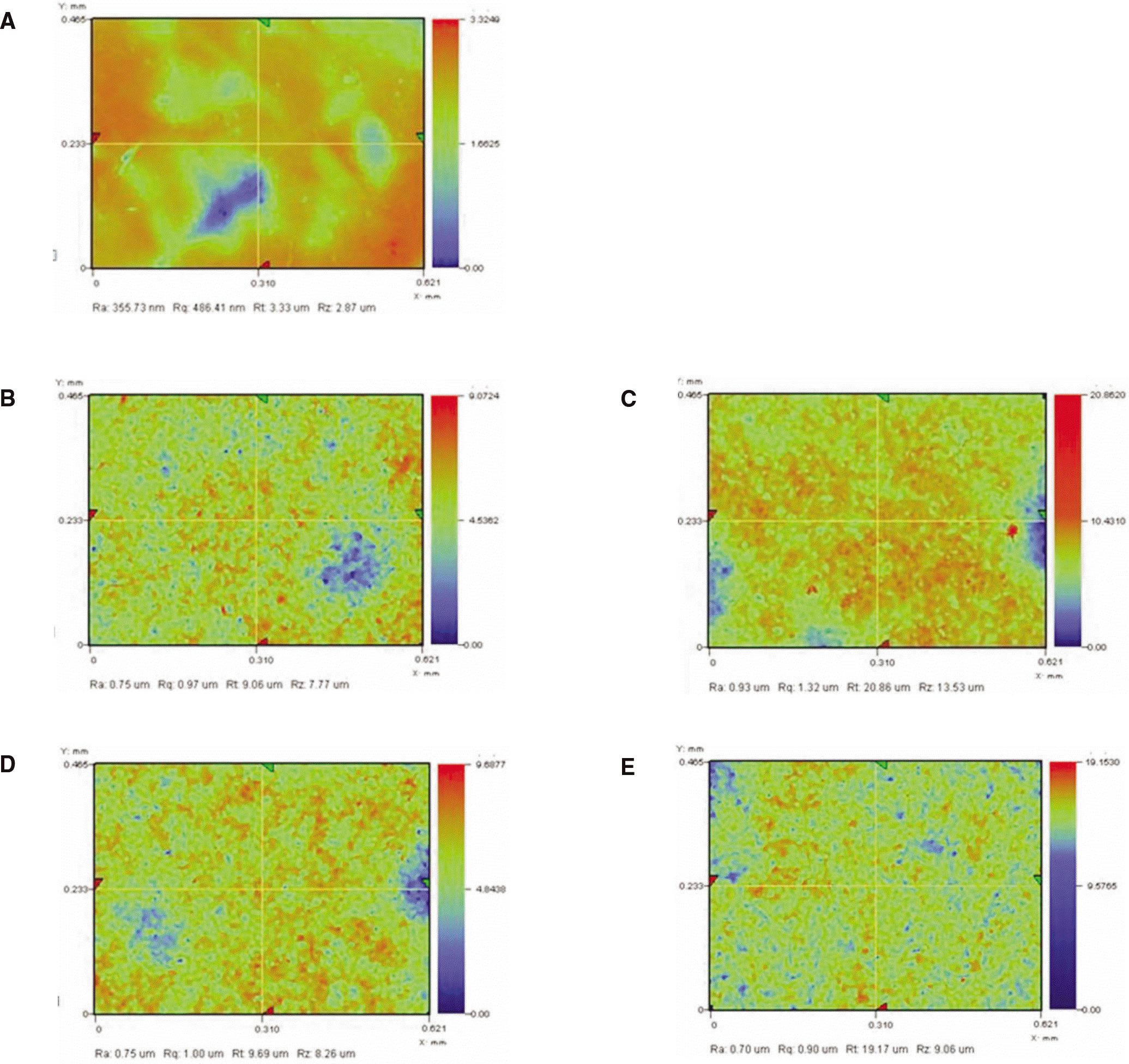 | Fig. 7.A: Surface roughness without sandblasting, B: Surface roughness after 30 sec sandblasting, C: Surface roughness after 1 min sandblasting, D: Surface roughness after 2 min sandblasting, E: Surface roughness after 4 min sandblasting. |
Table 1.
Materials used for this study
Table 2.
Experimental groups
Table 3.
Result of 1-way analysis of variance (group)
| Source | DF | Anova SS | Mean Square | F value | Pr > F |
|---|---|---|---|---|---|
| Group | 2 | 0.57 | 0.28 | 41.68 | < .0001 |
| Tukey grouping | Mean N | Group | |||
| α | 0.33 18 | B | |||
| β | 0.17 18 | C | |||
| γ | 0.08 18 | A | |||
Table 4.
Result of 1-way analysis of variance (weeks)
| Source | DF | Anova SS | Mean Square | F value | Pr > F |
|---|---|---|---|---|---|
| Wks | 2 | 0.08 | 0.04 | 2.30 | 0.1106 |
| Tukey grouping | Mean N | Wks | |||
| α | 0.24 18 | 0 | |||
| α | 0.19 18 | 1 | |||
| α | 0.15 18 | 2 | |||
Table 5.
Result of 2-way analysis of variance
Table 6.
Result of 1-way analysis of variance (group A)
| Source | DF | Anova SS | Mean Square | F value | Pr > F |
|---|---|---|---|---|---|
| Wks | 2 | 0.04 | 0.02 | 17.16 | 0.0001 |
| Tukey grouping | Mean N | Wks | |||
| α | 0.13 6 | 0 | |||
| β | 0.08 6 | 1 | |||
| γ | 0.03 6 | 2 | |||




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download