Abstract
Purpose
The aim of this in vitro study was to estimate surface characteristic after peptide coating and investigate biological response of human mesenchymal stem cell to anodized titanium discs coated with RGD peptide by physical adhesion and chemical fixation.
Materials and methods
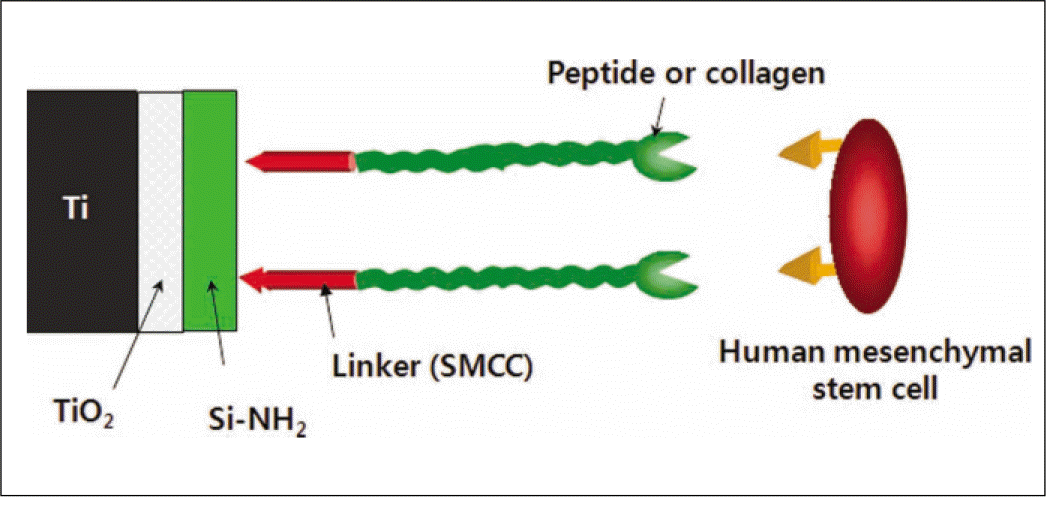
Fluorescence isothiocyanate (FITC) modified RGD-peptide was coated on the anodized titanium discs (diameter 12 mm, height 3 mm) using two methods. One was physical adhesion method and the other was chemical fixation method. Physical adhesion was performed by dip and dry procedure, chemical fixation was performed by covalent bond via silanization. In this study, human mesenchymal stem cell was used for experiments. The experiments consisted of surface characteristic evaluation after peptide coating, analysis about cell adhesion, proliferation, differentiation, and mineralization. Obtained data are statistically treated using Kruskal-Wallis test and Bonferroni test was performed as post hoc test (P=.05).
Results
The evaluation of FE-SEM images revealed no diffenrence at micro-surfaces between each groups. Total coating dose was higher at physical adhesion experimental group than at chemical fixation experimental group. In cell adhesion and proliferation, RGD peptide coating did not show a statistical significance compared with control group (P>.05). In cell differentiation and mineralization, physical adhesion method displayed significantly increased levels compared with control group and chemical fixation method (P<.05).
Go to : 
REFERENCES
1.Le Gue′hennec L., Soueidan A., Layrolle P., Amouriq Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent Mater. 2007. 23:844–54.
2.Junker R., Dimakis A., Thoneick M., Jansen JA. Effects of implant surface coatings and composition on bone integration: a systematic review. Clin Oral Implants Res. 2009. 20:185–206.

3.Puleo DA., Nanci A. Understanding and controlling the bone-implant interface. Biomaterials. 1999. 20:2311–21.

4.Dettin M., Conconi MT., Gambaretto R., Bagno A., Di Bello C., Menti AM., Grandi C., Parnigotto PP. Effect of synthetic peptides on osteoblast adhesion. Biomaterials. 2005. 26:4507–15.

5.Kim KH., Kwon TY., Kim SY., Kang IK., Kim S., Yang Y., Ong JL. Preparation and characterization of anodized titanium surfaces and their effect on osteoblast responses. J Oral Implantol. 2006. 32:8–13.

6.Balasundaram G., Yao C., Webster TJ. TiO2 nanotubes functionalized with regions of bone morphogenetic protein-2 increases osteoblast adhesion. J Biomed Mater Res A. 2008. 84:447–53.
7.Kataoka Y., Tamaki Y., Miyazaki T. Synergistric responses of su-perficail chemistry and micro topography of titanium created by wire-type electric discharge machining. Biomed Mater Eng. 2011. 21113–21.
8.Sato M., Webster TJ. Nanobiotechnology: implications for the future of nanotechnology in orthopedic applications. Expert Rev Med Devices. 2004. 1:105–14.

9.Dard M., Sewing A., Meyer J., Verrier S., Roessler S., Scharnweber D. Tools for tissue engineering of mineralized oral structures. Clin Oral Investig. 2000. 4:126–9.

10.Ruoslahti E., Pierschbacher MD. Arg-Gly-Asp: a versatile cell recognition signal. Cell. 1986. 44:517–8.

11.Xiao SJ., Textor M., Spencer ND. Covalent attachment of cell-adhesive, (Arg-Gly-Asp)-containing peptides to titanium surfaces. Langmuir. 1998. 14:5507–16.

12.Massia SP., Hubbell JA. Covalently attached GRGD on polymer surfaces promotes biospecific adhesion of mammalian cells. Ann N Y Acad Sci. 1990. 589:261–70.

13.Rezania A., Johnson R., Lefkow AR., Healy KE. Bioactivation of metal oxide surfaces. 1. Surface characterization and cell response. Langmuir. 1999. 15:6931–9.

14.Grzesik WJ., Robey PG. Bone matrix RGD glycoproteins: im-munolocalization and interaction with human primary osteoblastic bone cells in vitro. J Bone Miner Res. 1994. 9:487–96.

15.Schaffner P., Meyer J., Dard M., Wenz R., Nies B., Verrier S., Kessler H., Kantlehner M. Induced tissue integration of bone implants by coating with bone selective RGD-peptides in vitro and in vivo studies. J Mater Sci Mater Med. 1999. 10:837–9.
16.Kantlehner M., Schaffner P., Finsinger D., Meyer J., Jonczyk A., Diefenbach B., Nies B., Ho ¨lzemann G., Goodman SL., Kessler H. Surface coating with cyclic RGD peptides stimulates osteoblast adhesion and proliferation as well as bone formation. Chembiochem. 2000. 1:107–14.

17.Piattelli A., Scarano A., Corigliano M., Piattelli M. Effects of alkaline phosphatase on bone healing around plasma-sprayed titanium implants: a pilot study in rabbits. Biomaterials. 1996. 17:1443–9.

18.Lind M., Overgaard S., Ongpipattanakul B., Nguyen T., Bu ¨nger C., S � balle K. Transforming growth factor-beta 1 stimulates bone on-growth to weight-loaded tricalcium phosphate coated implants: an experimental study in dogs. J Bone Joint Surg Br. 1996. 78:377–82.
19.Liu SQ., Ito Y., Imanishi Y. Cell growth on immobilized cell growth factor: 5. Interaction of immobilized transferrin with fibroblast cells. Int J Biol Macromol. 1993. 15:221–6.

20.Kilpadi KL., Sawyer AA., Prince CW., Chang PL., Bellis SL. Primary human marrow stromal cells and Saos-2 osteosarcoma cells use different mechanisms to adhere to hydroxylapatite. J Biomed Mater Res A. 2004. 68:273–85.

21.Rezania A., Healy KE. The effect of peptide surface density on mineralization of a matrix deposited by osteogenic cells. J Biomed Mater Res. 2000. 52:595–600.

22.Zreiqat H., Akin FA., Howlett CR., Markovic B., Haynes D., Lateef S., Hanley L. Differentiation of human bone-derived cells grown on GRGDSP-peptide bound titanium surfaces. J Biomed Mater Res A. 2003. 64:105–13.

23.Huang H., Zhao Y., Liu Z., Zhang Y., Zhang H., Fu T., Ma X. Enhanced osteoblast functions on RGD immobilized surface. J Oral Implantol. 2003. 29:73–9.

Go to : 
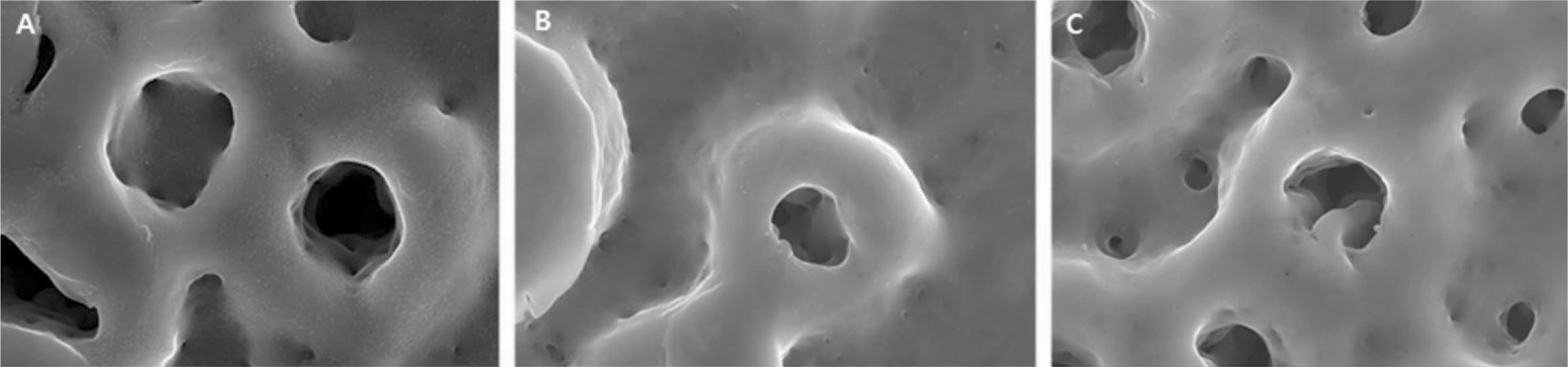 | Fig. 2.FE-SEM images after peptide coating (×50,000 magnification). A: Control, B: Chemical fixation, C: Physical adhesion. |
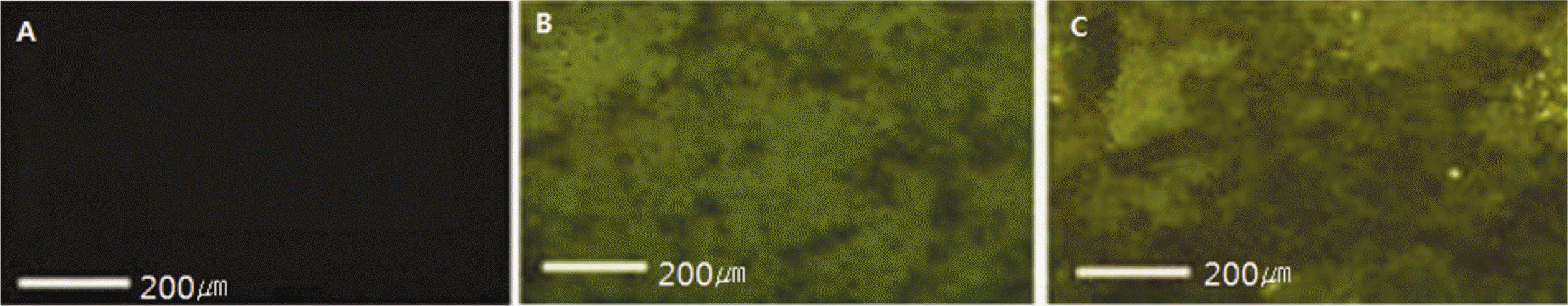 | Fig. 3.Fluorescent microscope images after peptide coating. A: Control, B: Chemical fixation, C: Physical adhesion. |
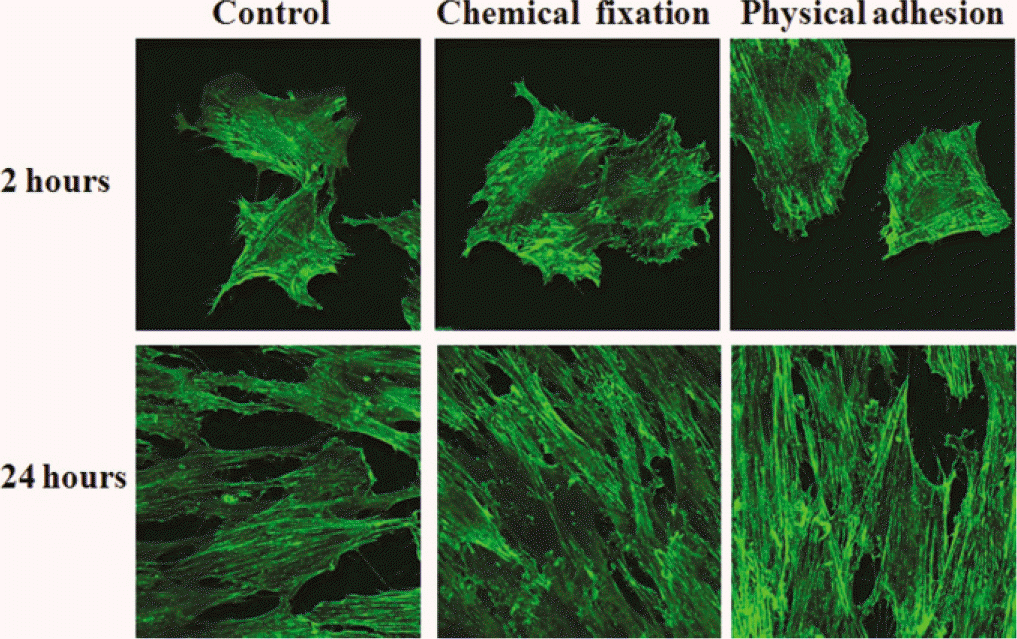 | Fig. 5.Confocal laser scanning microscopy images of cells grown on the surface of titanium specimen after incubation of 2 hours and 24 hours (×400 magnification). actin staining. |
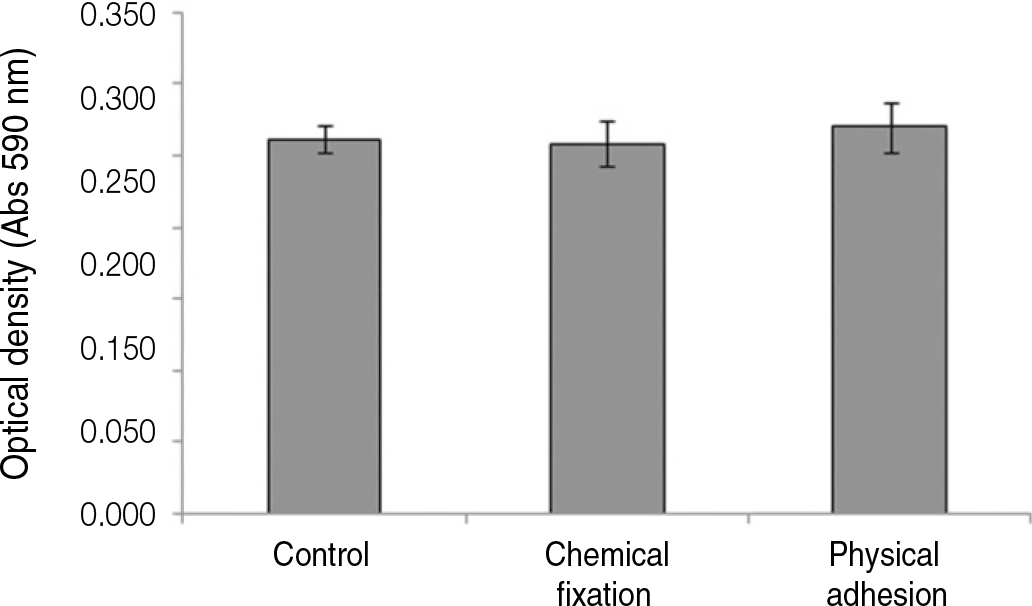 | Fig. 6.Crystal violet assay of human mesenchymal stem cells grown on the surface of titanium specimen after incubation of 3 hours. |
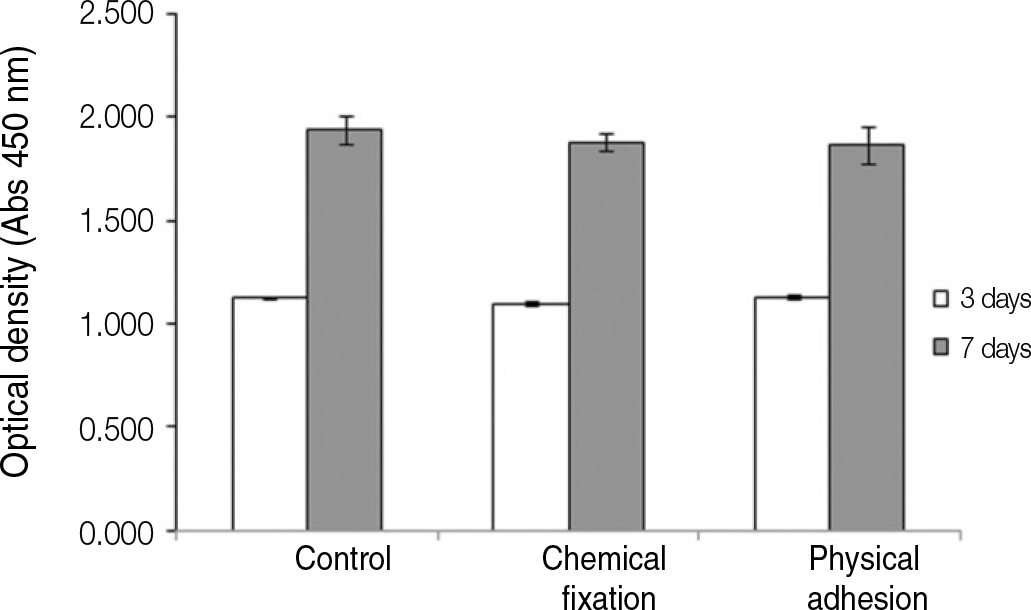 | Fig. 7.Cell proliferation of human mesenchymal stem cells grown on the surface of titanium specimen after incubation of 3 days and 7 days. |
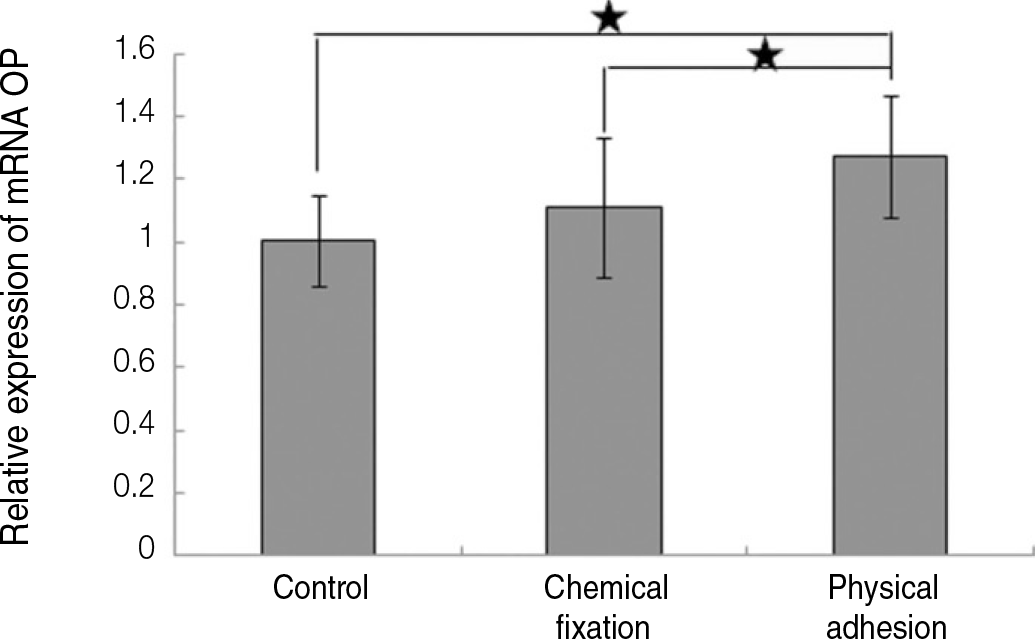 | Fig. 8.Real time-PCR analysis for mRNA of osteopontin grown on the surface of titanium specimen after incubation of 7 days (★:P<.05, Bonferroni test). |
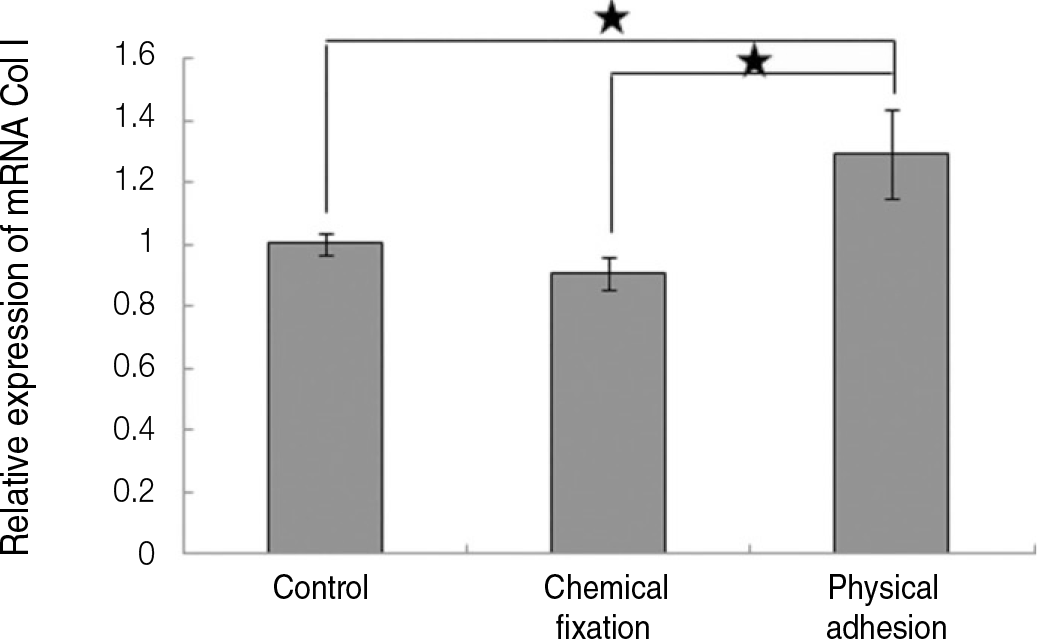 | Fig. 9.Real time-PCR analysis for mRNA of collagen type I grown on the surface of titanium specimen after incubation of 7 days (★:P<.05, Bonferroni test). |
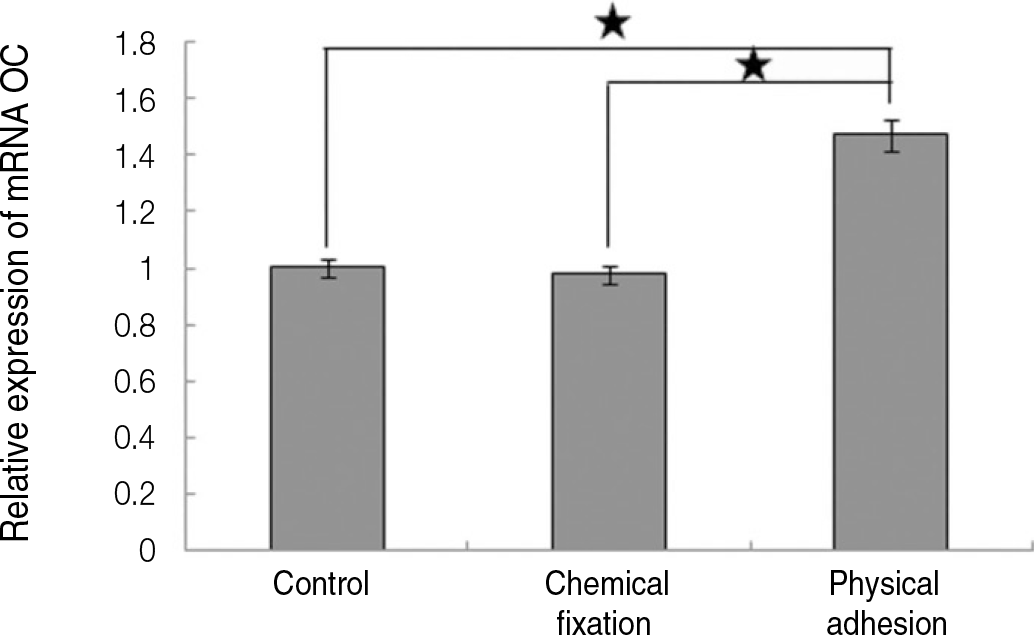 | Fig. 10.Real time-PCR analysis for mRNA of osteocalcin grown on the surface of titanium specimen after incubation of 7 days (★:P<.05, Bonferroni test). |
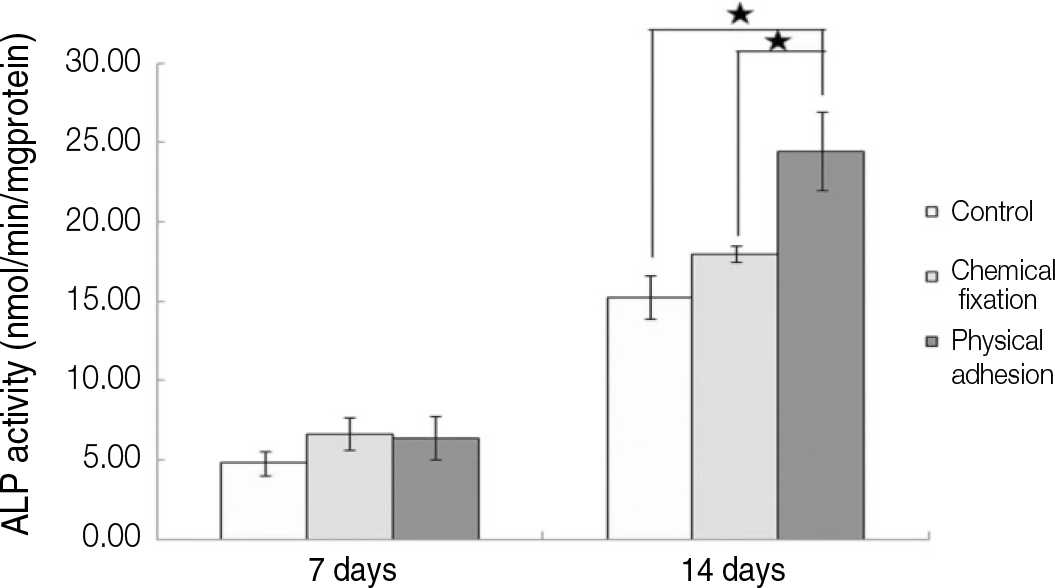 | Fig. 11.ALP activity of humen mesenchymal stem cells grown on the surface of titanium specimen after incubation of 7 days and 14 days (★:P<.05, Bonferroni test). |
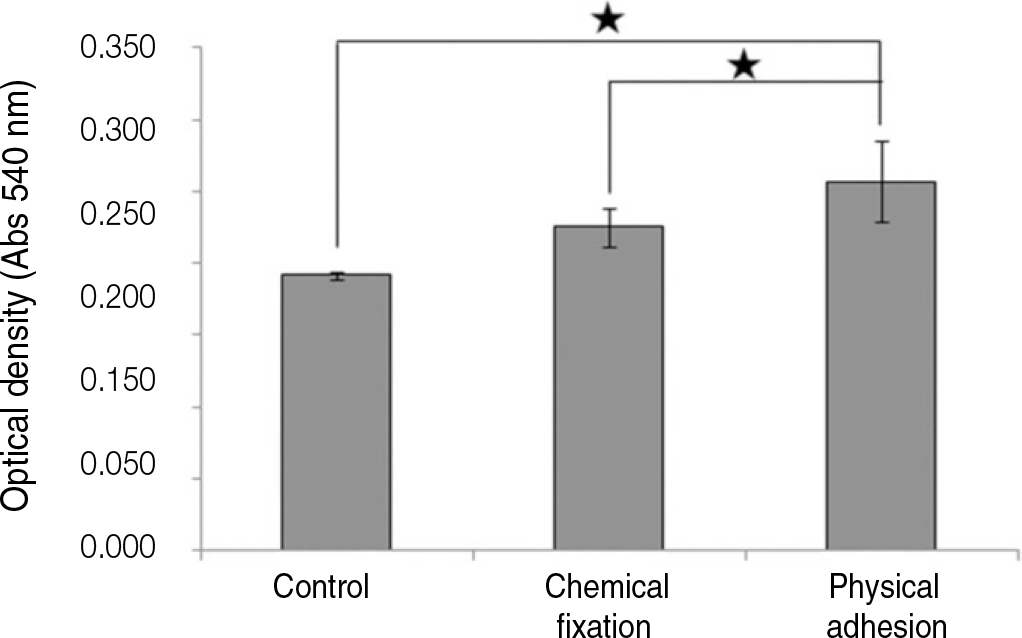 | Fig. 12.Alizarin red assay of humen mesenchymal cells grown on the surface of titanium specimen after incubation of 21 days (★: P<.05, Bonferroni test). |
Table 1.
RT-PCR primer sequence of bone forming related gene




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download