Abstract
Purpose
The purpose of this study was to investigate the bioactivity of precalcified nanotubular TiO2 layer on Ti-6Al-7Nb alloy.
Materials and methods
Anodic oxidation was carried out at a potential of 20 V and current density of 20 mA/cm2 for 1 hour. The glycerol solution containing 1 wt% NH4F and 20 wt% deionized water was used as an electrolyte. Precalcification treatment was obtained by soaking in Na2HPO4 solution at 80℃ for 30 minutes followed by soaking in saturated Ca(OH)2 solution at 100℃ for 30 minutes, followed by heat treatment at 500℃ for 2 hours. To evaluate the activity of precalcified nanotubular TiO2 layer, specimens were immersed in a simulated body fluid with pH 7.4 at 36.5℃ for 10 days.
Results
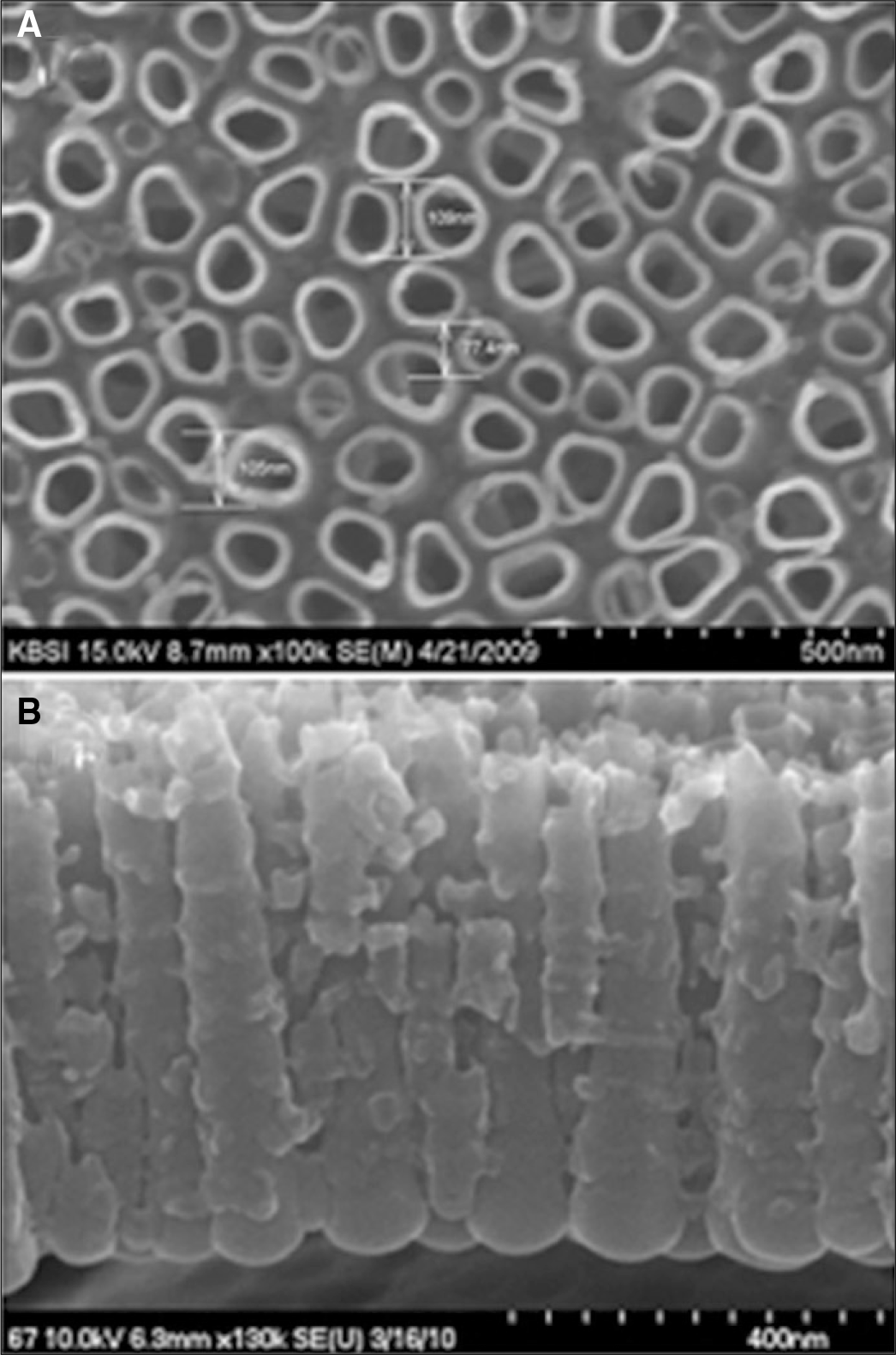
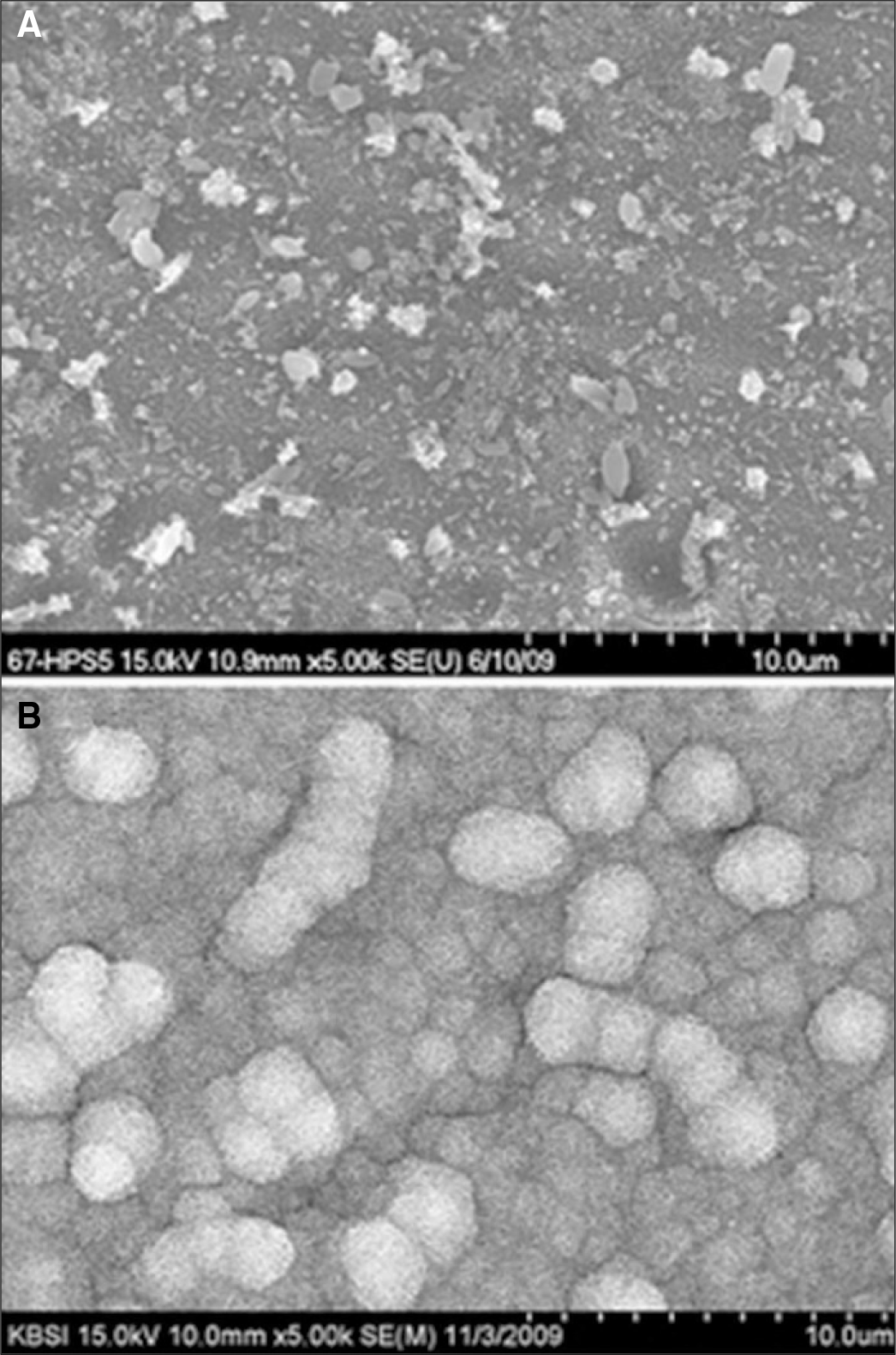
1. Nanotubular TiO2 layer showed the highly ordered dense structure by interposing small diameter nanotubes between large ones, the shape of nanotubes was enlarged as going down. 2. The mean length of nanotubes was 517.0 ± 23.2 nm innm glycerol solution containing 1 wt% NH4F and 20 wt% H2O at 20 V for 1 hour. 3. The bioactivity of Ti-6Al-7Nb alloy was improved with formation of nanotubular TiO2 layer and precalcification treatment in 80℃ 0.5 M Na2HPO4 and saturated 100℃ Ca(OH)2 solution.
Go to : 
REFERENCES
1.Kasemo B. Lausmaa J. Metal selection and surface characteristics. In: Bra®nemark PI, Zarb GA, Albrektsson T (eds), Tissue-integrated prostheses, Osseointegrated in clinical dentistry. Quintessence: Chicago;1985. p. 99–116.
2.Hanawa T., Ukai H., Murakami K., Asaoka K. Structure of Surface-Modified Layers of Calcium-Ion-Implanted Ti-6Al-4V and Ti-56Ni. Mater Trans JIM. 1995. 36:438–44.
3.Hanawa T., Asami K., Asaoka K. Microdissolution of calcium ions from calcium-ion-implanted titanium. Corros Sci. 1996. 38:1579–94.

4.Wen HB., Wolke JG., de Wijn Jr., Liu Q., Cui FZ., de Groot K. Fast precipitation of calcium phosphate layers on titanium induced by simple chemical treatments. Biomaterials. 1997. 18:1471–8.

5.Cheang P., Khor KA. Addressing processing problems associated with plasma spraying of hydroxyapatite coatings. Biomaterials. 1996. 17:537–44.

6.De Andrade MC., Sader MS., Filgueiras MR., Ogasawara T. Microstructure of ceramic coating on titanium surface as a result of hydrothermal treatment. J Mater Sci Mater Med. 2000. 11:751–5.
7.Wang J., Layrolle P., Stigter M., de Groot K. Biomimetic and electrolytic calcium phosphate coatings on titanium alloy: physicochemical characteristics and cell attachment. Biomaterials. 2004. 25:583–92.

8.Larsson C., Thomsen P., Aronsson BO., Rodahl M., Lausmaa J., Kasemo B., Ericson LE. Bone response to surface-modified titanium implants: studies on the early tissue response to machined and electropolished implants with different oxide thicknesses. Biomaterials. 1996. 17:605–16.

9.Kim SW., Yoon IH., Choe HC., Ko YM. Effects of surface roughness on the electrochemical characteristics of cell cultured Ti-6Al-4V alloy. J Korean Res Soc Dent Materials. 2005. 32:303–12.
10.Ishizawa H., Ogino M. Characterization of thin hydroxyapatite layers formed on anodic titanium oxide films containing Ca and P by hydrothermal treatment. J Biomed Mater Res. 1995. 29:1071–9.

11.Neupane MP., Kim YK., Park IS., Kim KA., Lee MH., Bae TS. Temperature driven morphological changes of hydrothermally prepared copper oxide nanoparticles. Surf Interface Anal. 2009. 41:259–63.

12.Ma Q., Li M., Hu Z., Chen Q., Hu W. Enhancement of the bioactivity of titanium oxide nanotubes by precalcification. Mater Lett. 2008. 62:3035–8.

13.Rupp F., Scheideler L., Olshanska N., de Wild M., Wieland M., Geis-Gerstorfer J. Enhancing surface free energy and hydrophilicity through chemical modification of microstructured titanium implant surfaces. J Biomed Mater Res A. 2006. 76:323–34.

14.Ellingsen JE., Johansson CB., Wennerberg A., Holme′n A. Improved retention and bone-tolmplant contact with fluoride-modified titanium implants. Int J Oral Maxillofac Implants. 2004. 19:659–66.
15.Hirata T., Nakamura T., Takashima F., Maruyama T., Taira M., Takahashi J. Studies on polishing of Ti and Ag-Pd-Cu-Au alloy with five dental abrasives. J Oral Rehabil. 2001. 28:773–7.

16.Kuroiwa A., Igarashi Y. Application of pure titanium to metal framework. J Jpn Prosthodont Soc. 1998. 42:547–58.

17.Cai Z., Shafer T., Watanabe I., Nunn ME., Okabe T. Electrochemical characterization of cast titanium alloys. Biomaterials. 2003. 24:213–8.

18.Iijima D., Yoneyama T., Doi H., Hamanaka H., Kurosaki N. Wear properties of Ti and Ti-6Al-7Nb castings for dental prostheses. Biomaterials. 2003. 24:1519–24.

19.Wang K. The use of titanium for medical applications in the USA. Mater Sci Eng A. 1996. 213:134–7.

20.Eisenbarth E., Velten D., Mu ¨ller M., Thull R., Breme J. Biocompatibility of beta-stabilizing elements of titanium alloys. Biomaterials. 2004. 25:5705–13.
21.Kaneco S., Chen Y., Westerhoff P., Crittenden JC. Fabrication of uniform size titanium oxide nanotubes: Impact of current density and solution conditions. J Scripta Mat. 2007. 56:373–6.

22.Macak JM., Tsuchiya H., Ghicov A., Yasuda K., Hahn R., Bauer S., Schmuki P. TiO2 nanotubes: Self-organized electrochemical formation, properties and applications. Curr Opin Solid State Mater Sci. 2007. 11:3–18.

23.Beranek R., Hidebrand H., Schmuki P. Self-Organized Porous Titanium Oxide Prepared in H2SO4/HF Electrolytes. Electrochem Solid-State Lett. 2003. 6:B12–4.
24.Kunze J., Mu ¨ller L., Macak JM., Greil P., Schmuki P., Mu ¨ller FA. Time-dependent growth of biomimetic apatite on anodic TiO2 nanotubes. Electrochimica Acta. 2008. 53:6995–7003.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download