Abstract
Purpose
This study was aimed to evaluate the effect of different sizes of β-TCP/HA particles on vertical bone augmentation using titanium mesh in the cranium of rabbits.
Material and methods
Six white rabbits weighing 5kg were used. Four circular grooves of 6mm diameter were made by trephine, and five small holes were drilled in the inner surface of each circular gooves. Different sizes of grafts (small 0.3 - 0.5 mm, medium 0.5 - 1.0, large 1.0 - 2.0 mm) were placed respectively in the experimental groups. Titanium mesh (height 3 mm, width 6 mm) was placed. After 8weeks healing period, the rabbits were euthanized, and the specimens were prepared for histological findings. New bone formation and remaining graft area were measured to calculate the ratio of areas occupying the inner space of titanium mesh. Mann-Whitney U-test and Wilcoxon signed rank-test were used for statistical analysis (α = .05).
Results
The experimental groups with β-TCP/HA graft showed a significantly higher new bone formation (P = .003). Comparing different sizes of β-TCP/HA, there was no statistical difference in terms of new bone formation. The vertical bone formation (i.e. new bone and graft area) was significantly greater in β-TCP/HA groups (P = .001). In comparison between different sizes of β-TCP/HA, medium size group had significantly greater area than large particle size group (P = .039).
Figures and Tables
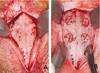 | Fig. 1A: A flap was made using a midsagittal incision and exfoliated from the forehead. The periosteum was incised and lifted to expose the cranium on both sides of the midline, B: A circular groove was prepared on each side of the midline using a trephine drill. Five small holes were drilled with 1mm diameter round bur to induce bleeding from the bone marrow space within the circle. |
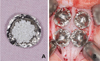 | Fig. 2A: The experimental titanium mesh caps were filled with β-TCP/HA as the experimental site or without β-TCP as the control site, B: Fixation of meshes including β-TCP/HP by titanium pins. |
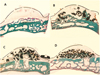 | Fig. 3Sagittal histological section of the titanium mesh (×12.5).
A : control group, no β-TCP/HA, newly generated tissue reaches to almost the height of the titanium mesh and there is a little newly formed bone. B (large particle size), C (medium particle size), D (small particle size): experimental groups, containing β-TCP/HA, Newly generated tissue, newly formed bone has formed surrounding β-TCP/HA. β-TCP/HAP particles remain in the upper part of the titanium mesh. there is more newly formed bone than in control group. In large particle size, there are less β-TCP/HA particles than other experimental groups.
|
References
1. Dahlin C. Buser D, Dahlin C, Schenk RK, editors. Scientific background of guided bone regeneration. Guided bone regeneration in implant dentistry. 1994. Hong Kong: Quintessence;31–48.
2. Dahlin C, Sennerby L, Lekholm U, Linde A, Nyman S. Generation of new bone around titanium implants using a membrane technique: an experimental study in rabbits. Int J Oral Maxillofac Implants. 1989. 4:19–25.
3. Becker W, Becker BE, Handlesman M, Celletti R, Ochsenbein C, Hardwick R, Langer B. Bone formation at dehisced dental implant sites treated with implant augmentation material: a pilot study in dogs. Int J Periodontics Restorative Dent. 1990. 10:92–101.
4. Schenk RK, Buser D, Hardwick WR, Dahlin C. Healing pattern of bone regeneration in membrane-protected defects: a histologic study in the canine mandible. Int J Oral Maxillofac Implants. 1994. 9:13–29.
5. Kostopoulos L, Karring T. Guided bone regeneration in mandibular defects in rats using a bioresorbable polymer. Clin Oral Implants Res. 1994. 5:66–74.

6. Kostopoulos L, Karring T. Augmentation of the rat mandible using guided tissue regeneration. Clin Oral Implants Res. 1994. 5:75–82.

7. Kostopoulos L, Karring T, Uraguchi R. Formation of jawbone tuberosities by guided tissue regeneration. An experimental study in the rat. Clin Oral Implants Res. 1994. 5:245–253.

8. Lioubavina N, Kostopoulos L, Wenzel A, Karring T. Long-term stability of jaw bone tuberosities formed by "guided tissue regeneration". Clin Oral Implants Res. 1999. 10:477–486.

9. Nyman S, Lang NP, Buser D, Bragger U. Bone regeneration adjacent to titanium dental implants using guided tissue regeneration: a report of two cases. Int J Oral Maxillofac Implants. 1990. 5:9–14.
10. Buser D, Dula K, Belser U, Hirt HP, Berthold H. Localized ridge augmentation using guided bone regeneration. 1. Surgical procedure in the maxilla. Int J Periodontics Restorative Dent. 1993. 13:29–45.
11. Hämmerle CH, Karring T. Guided bone regeneration at oral implant sites. Periodontol 2000. 1998. 17:151–175.

12. Linde A, Thorén C, Dahlin C, Sandberg E. Creation of new bone by an osteopromotive membrane technique: an experimental study in rats. J Oral Maxillofac Surg. 1993. 51:892–897.

13. Schmid J, Hämmerle CH, Stich H, Lang NP. Supraplant, a novel implant system based on the principle of guided bone generation. A preliminary study in the rabbit. Clin Oral Implants Res. 1991. 2:199–202.

14. Buser D, Brägger U, Lang NP, Nyman S. Regeneration and enlargement of jaw bone using guided tissue regeneration. Clin Oral Implants Res. 1990. 1:22–32.

15. Schmid J, Hämmerle CH, Flückiger L, Winkler JR, Olah AJ, Gogolewski S, Lang NP. Blood-filled spaces with and without filler materials in guided bone regeneration. A comparative experimental study in the rabbit using bioresorbable membranes. Clin Oral Implants Res. 1997. 8:75–81.

16. Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. Complications of iliac crest bone graft harvesting. Clin Orthop Relat Res. 1996. 329:300–309.

17. Goulet JA, Senunas LE, DeSilva GL, Greenfield ML. Autogenous iliac crest bone graft. Complications and functional assessment. Clin Orthop Relat Res. 1997. 339:76–81.

18. Wippermann BW, Schratt HE, Steeg S, Tscherne H. Complications of spongiosa harvesting of the ilial crest. A retrospective analysis of 1,191 cases. Chirurg. 1997. 68:1286–1291.

19. Ha JW, Jung HJ. Preparation of dense polycrystalline hydroxyapatite ceramics for the application of tooth implants. J Korean Ceram Soc. 1983. 20:55–62.
20. Jarcho M. Calcium phosphate ceramics as hard tissue prosthetics. Clin Orthop Relat Res. 1981. 157:259–278.

21. Yukna RA. Osseous defect responses to hydroxylapatite grafting versus open flap debridement. J Clin Periodontol. 1989. 16:398–402.

22. Bowers GM, Vargo JW, Levy B, Emerson JR, Bergquist JJ. Histologic observations following the placement of tricalcium phosphate implants in human intrabony defects. J Periodontol. 1986. 57:286–287.

23. Saffar JL, Colombier ML, Detienville R. Bone formation in tricalcium phosphate-filled periodontal intrabony lesions. Histological observations in humans. J Periodontol. 1990. 61:209–216.

24. Zaner DJ, Yukna RA. Particle size of periodontal bone grafting materials. J Periodontol. 1984. 55:406–409.

25. Mellonig JT. Polosn AM, editor. Osseous grafts and periodontal regeneration. Periodontal regeneration-Current Status and Direction. 1994. Berlin: Quintessence.
26. Fucini SE, Quintero G, Gher ME, Black BS, Richardson AC. Small versus large particles of demineralized freeze-dried bone allografts in human intrabony periodontal defects. J Periodontol. 1993. 64:844–847.

27. Murai M, Sato S, Fukase Y, Yamada Y, Komiyama K, Ito K. Effects of different sizes of beta-tricalcium phosphate particles on bone augmentation within a titanium cap in rabbit calvarium. Dent Mater J. 2006. 25:87–96.

28. Fucini SE, Quintero G, Gher ME, Black BS, Richardson AC. Small versus large particles of demineralized freeze-dried bone allografts in human intrabony periodontal defects. J Periodontol. 1993. 64:844–847.

29. Kon K, Shiota M, Ozeki M, Yamashita Y, Kasugai S. Bone augmentation ability of autogenous bone graft particles with different sizes: a histological and micro-computed tomography study. Clin Oral Implants Res. 2009. 20:1240–1246.

30. Xu H, Shimizu Y, Asai S, Ooya K. Experimental sinus grafting with the use of deproteinized bone particles of different sizes. Clin Oral Implants Res. 2003. 14:548–555.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download