Abstract
Purpose
The aim of this experimental study is to observe the effect of platelet-rich plasma (PRP) on early bone regeneration of rats both in normal condition and in osteoporosis induced by ovariectomy.
Material and methods
Total 40 Sprague-Dawley female rats were divided into 4 groups. A 8-mm-diameter calvarial critical-sized defect (CSD) was made by drilling with trephine at the center of calvaria in cranium of every rat. Every CSD was augmented with an osteoconductive synthetic alloplastic substitute (MBCPTM) and PRP as follows. Group A; 10 non-ovariectomized rats grafted with only MBCPTM. Group B; 10 non-ovariectomized rats grafted with MBCPTM and PRP. Group C; 10 ovariectomized rats grafted with only MBCPTM. Group D; 10 ovariectomized rats grafted with MBCPTM and PRP. At 4 weeks after graft, every rat was sacrificed. And histomorphometric analysis was performed by measuring calcified area of new bone formation within the CSD.
Results
Comparing four groups, results were obtained as follows. 1. In non-ovariectomized groups, PRP showed a positive effect somewhat but not significant (P > .05). 2. In ovariectomized groups, PRP showed a positive effect significantly (P < .05). 3. In PRP untreated groups, ovariectomy diminished bone regeneration significantly (P < .05). 4. In PRP treated groups, ovariectomy diminished bone regeneration somewhat but not significant (P > .05).
Conclusion
Within the limitation of this study, it can be concluded that PRP in combination with an osteoconductive synthetic alloplastic substitute has an effect on bone regeneration more significantly in ovariectomized osteoporotic rats than in normal rats. (J Korean Acad Prosthodont 2010;48:16-27)
Go to : 
REFERENCES
1.Lynch SE., de Castilla GR., Williams RC., Kiritsy CP., Howell TH., Reddy MS., Antoniades HN. The effects of short-term application of a combination of platelet-derived and insulin-like growth factors on periodontal wound healing. J Periodontol. 1991. 62:458–67.

2.Terranova VP., Odziemiec C., Tweden KS., Spadone DP. Repopulation of dentin surfaces by periodontal ligament cells and endothelial cells. Effect of basic fibroblast growth factor. J Periodontol. 1989. 60:293–301.
3.Kiritsy CP., Lynch AB., Lynch SE. Role of growth factors in cutaneous wound healing: a review. Crit Rev Oral Biol Med. 1993. 4:729–60.

5.Kenley RA., Yim K., Abrams J., Ron E., Turek T., Marden LJ., Hollinger JO. Biotechnology and bone graft substitutes. Pharm Res. 1993. 10:1393–401.
6.Sculean A., Chiantella GC., Windisch P., Donos N. Clinical and histologic evaluation of human intrabony defects treated with an enamel matrix protein derivative (Emdogain). Int J Periodontics Restorative Dent. 2000. 20:374–81.
7.Jiang D., Dziak R., Lynch SE., Stephan EB. Modification of an osteoconductive anorganic bovine bone mineral matrix with growth factors. J Periodontol. 1999. 70:834–9.

8.Roberts AB., Sporn MB. Physiological actions and clinical applications of transforming growth factor-beta (TGF-beta). Growth Factors. 1993. 8:1–9.
9.Aspenberg P., Albrektsson T., Thorngren KG. Local application of growth-factor IGF-1 to healing bone. Experiments with a titanium chamber in rabbits. Acta Orthop Scand. 1989. 60:607–10.

10.Marx RE., Carlson ER., Eichstaedt RM., Schimmele SR., Strauss JE., Georgeff KR. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998. 85:638–46.
11.Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004. 62:489–96.

12.Sa ′nchez AR., Sheridan PJ., Kupp LI. Is platelet-rich plasma the perfect enhancement factor? A current review. Int J Oral Maxillofac Implants. 2003. 18:93–103.
13.Aghaloo TL., Moy PK., Freymiller EG. Investigation of platelet-rich plasma in rabbit cranial defects: A pilot study. J Oral Maxillofac Surg. 2002. 60:1176–81.

16.Wergedal JE., Mohan S., Lundy M., Baylink DJ. Skeletal growth factor and other growth factors known to be present in bone matrix stimulate proliferation and protein synthesis in human bone cells. J Bone Miner Res. 1990. 5:179–86.

17.Yim SB., Lee KS., Park YC., You HG., Shin HS. The effect of platelet rich plasma combined with bovine bone on the treatment of grade II furcation defects in beagle dogs. J Korean Acad Periodontol. 2000. 30:257–78.

18.Wartiovaara U., Salven P., Mikkola H., Lassila R., Kaukonen J., Joukov V., Orpana A., Ristima ¨ki A., Heikinheimo M., Joensuu H., Alitalo K., Palotie A. Peripheral blood platelets express VEGF-C and VEGF which are released during platelet activation. Thromb Haemost. 1998. 80:171–5.

19.Anitua E. Plasma rich in growth factors: preliminary results of use in the preparation of future sites for implants. Int J Oral Maxillofac Implants. 1999. 14:529–35.
20.Lozada JL., Caplanis N., Proussaefs P., Willardsen J., Kammeyer G. Platelet-rich plasma application in sinus graft surgery: Part I-Background and processing techniques. J Oral Implantol. 2001. 27:38–42.

21.Kassolis JD., Rosen PS., Reynolds MA. Alveolar ridge and sinus augmentation utilizing platelet-rich plasma in combination with freeze-dried bone allograft: case series. J Periodontol. 2000. 71:1654–61.

22.Wiltfang J., Schlegel KA., Schultze-Mosgau S., Nkenke E., Zimmermann R., Kessler P. Sinus floor augmentation with beta-tricalciumphosphate (beta-TCP): does platelet-rich plasma promote its osseous integration and degradation? Clin Oral Implants Res. 2003. 14:213–8.
23.Rodriguez A., Anastassov GE., Lee H., Buchbinder D., Wettan H. Maxillary sinus augmentation with deproteinated bovine bone and platelet rich plasma with simultaneous insertion of endosseous implants. J Oral Maxillofac Surg. 2003. 61:157–63.

24.Vercellotti T. Piezoelectric surgery in implantology: a case report—a new piezoelectric ridge expansion technique. Int J Periodontics Restorative Dent. 2000. 20:358–65.
25.Fennis JP., Stoelinga PJ., Jansen JA. Mandibular reconstruction: a histological and histomorphometric study on the use of autogenous scaffolds, particulate cortico-cancellous bone grafts and platelet rich plasma in goats. Int J Oral Maxillofac Surg. 2004. 33:48–55.

26.Fennis JP., Stoelinga PJ., Jansen JA. Mandibular reconstruction: a clinical and radiographic animal study on the use of autogenous scaffolds and platelet-rich plasma. Int J Oral Maxillofac Surg. 2002. 31:281–6.

27.Camargo PM., Lekovic V., Weinlaender M., Vasilic N., Madzarevic M., Kenney EB. Platelet-rich plasma and bovine porous bone mineral combined with guided tissue regeneration in the treatment of intrabony defects in humans. J Periodontal Res. 2002. 37:300–6.

28.Lekovic V., Camargo PM., Weinlaender M., Vasilic N., Aleksic Z., Kenney EB. Effectiveness of a combination of platelet-rich plasma, bovine porous bone mineral and guided tissue regeneration in the treatment of mandibular grade II molar furcations in humans. J Clin Periodontol. 2003. 30:746–51.

29.de Obarrio JJ., Arau ′z-Dutari JI., Chamberlain TM., Croston A. The use of autologous growth factors in periodontal surgical therapy: platelet gel biotechnology-case reports. Int J Periodontics Restorative Dent. 2000. 20:486–97.
30.Choi BH., Im CJ., Huh JY., Suh JJ., Lee SH. Effect of platelet-rich plasma on bone regeneration in autogenous bone graft. Int J Oral Maxillofac Surg. 2004. 33:56–9.

31.Wiltfang J., Kloss FR., Kessler P., Nkenke E., Schultze-Mosgau S., Zimmermann R., Schlegel KA. Effects of platelet-rich plasma on bone healing in combination with autogenous bone and bone substitutes in critical-size defects. An animal experiment. Clin Oral Implants Res. 2004. 15:187–93.
32.Kim SG., Kim WK., Park JC., Kim HJ. A comparative study of osseointegration of Avana implants in a demineralized freeze-dried bone alone or with platelet-rich plasma. J Oral Maxillofac Surg. 2002. 60:1018–25.

33.Kim ES., Park EJ., Choung PH. Platelet concentration and its effect on bone formation in calvarial defects: an experimental study in rabbits. J Prosthet Dent. 2001. 86:428–33.

34.Aghaloo TL., Moy PK., Freymiller EG. Evaluation of platelet-rich plasma in combination with anorganic bovine bone in the rabbit cranium: a pilot study. Int J Oral Maxillofac Implants. 2004. 19:59–65.
35.Schlegel KA., Donath K., Rupprecht S., Falk S., Zimmermann R., Felszeghy E., Wiltfang J. De novo bone formation using bovine collagen and platelet-rich plasma. Biomaterials. 2004. 25:5387–93.

36.Whitman DH., Berry RL., Green DM. Platelet gel: an autologous alternative to fibrin glue with applications in oral and maxillofacial surgery. J Oral Maxillofac Surg. 1997. 55:1294–9.

37.Whitman DH., Berry RL. A technique for improving the handling of particulate cancellous bone and marrow grafts using platelet gel. J Oral Maxillofac Surg. 1998. 56:1217–8.
38.Plachokova AS., van den Dolder J., Stoelinga PJ., Jansen JA. The bone regenerative effect of platelet-rich plasma in combination with an osteoconductive material in rat cranial defects. Clin Oral Implants Res. 2006. 17:305–11.

39.Fu ¨rst G., Gruber R., Tangl S., Zechner W., Haas R., Mailath G., Sanroman F., Watzek G. Sinus grafting with autogenous platelet-rich plasma and bovine hydroxyapatite. A histomorphometric study in minipigs. Clin Oral Implants Res. 2003. 14:500–8.
40.Plachokova AS., van den Dolder J., Stoelinga PJ., Jansen JA. Early effect of platelet-rich plasma on bone healing in combination with an osteoconductive material in rat cranial defects. Clin Oral Implants Res. 2007. 18:244–51.

41.Froum SJ., Wallace SS., Tarnow DP., Cho SC. Effect of platelet-rich plasma on bone growth and osseointegration in human maxillary sinus grafts: three bilateral case reports. Int J Periodontics Restorative Dent. 2002. 22:45–53.
42.Schaaf H., Streckbein P., Lendeckel S., Heidinger KS., Rehmann P., Boedeker RH., Howaldt HP. Sinus lift augmentation using autogenous bone grafts and platelet-rich plasma: radiographic results. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008. 106:673–8.

43.Schmitz JP., Hollinger JO. The biology of platelet-rich plasma. J Oral Maxillofac Surg. 2001. 59:1119–21.

44.Riggs BL., Khosla S., Melton LJ 3rd. A unitary model for involutional osteoporosis: estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging men. J Bone Miner Res. 1998. 13:763–73.

45.Acarturk TO., Hollinger JO. Commercially available demineralized bone matrix compositions to regenerate calvarial critical-sized bone defects. Plast Reconstr Surg. 2006. 118:862–73.

46.Schmitz JP., Hollinger JO. The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin Orthop Relat Res. 1986. 205:299–308.

47.Okamura A., Ayukawa Y., Iyama S., Koyano K. Effect of the difference of bone turnover on peri-titanium implant osteogenesis in ovariectomized rats. J Biomed Mater Res A. 2004. 70:497–505.

48.Ozawa S., Ogawa T., Iida K., Sukotjo C., Hasegawa H., Nishimura RD., Nishimura I. Ovariectomy hinders the early stage of bone-implant integration: histomorphometric, biomechanical, and molecular analyses. Bone. 2002. 30:137–43.

49.Cho P., Schneider GB., Krizan K., Keller JC. Examination of the bone-implant interface in experimentally induced osteoporotic bone. Implant Dent. 2004. 13:79–87.

50.Jakse N., Tangl S., Gilli R., Berghold A., Lorenzoni M., Eskici A., Haas R., Pertl C. Influence of PRP on autogenous sinus grafts. An experimental study on sheep. Clin Oral Implants Res. 2003. 14:578–83.
51.Park SI., Chung CH., Lim SB., Kim JK. Biologic effect of platelet rich plasma on the initial attachment, proliferation and cellular activity of osteoblast. J Korean Acad Periodontol. 2001. 31:513–29.
52.Riggs BL. Overview of osteoporosis. West J Med. 1991. 154:63–77.
53.von Wowern N., Klausen B., Kollerup G. Osteoporosis: a risk factor in periodontal disease. J Periodontol. 1994. 65:1134–8.
54.Hohlweg-Majert B., Schmelzeisen R., Pfeiffer BM., Schneider E. Significance of osteoporosis in craniomaxillofacial surgery: a review of the literature. Osteoporos Int. 2006. 17:167–79.

56.Kribbs PJ. Comparison of mandibular bone in normal and osteoporotic women. J Prosthet Dent. 1990. 63:218–22.

57.Kribbs PJ., Chesnut CH 3rd., Ott SM., Kilcoyne RF. Relationships between mandibular and skeletal bone in an osteoporotic population. J Prosthet Dent. 1989. 62:703–7.

58.Nagareddy PR., Lakshmana M. Withania somnifera improves bone calcification in calcium-deficient ovariectomized rats. J Pharm Pharmacol. 2006. 58:513–9.

59.Sozer V., Uzun H., Guner I., Aydin S., Yucel R., Karter Y., Simsek C., Kaya S., Yigit G., Simsek G. Bone metabolism in ovariectomized rats with induced hyperthyroidism: the effect of estrogen replacement. Chin J Physiol. 2006. 49:335–41.
60.Midy V., Ploue ¨t J. Vasculotropin/vascular endothelial growth factor induces differentiation in cultured osteoblasts. Biochem Biophys Res Commun. 1994. 199:380–6.

61.Shui C., Scutt A. Mild heat shock induces proliferation, alkaline phosphatase activity, and mineralization in human bone marrow stromal cells and Mg-63 cells in vitro. J Bone Miner Res. 2001. 16:731–41.
62.Augat P., Simon U., Liedert A., Claes L. Mechanics and mechano-biology of fracture healing in normal and osteoporotic bone. Osteoporos Int. 2005. 16:S36–43.

63.Akinci B., Bayraktar F., Saklamaz A., Demir T., Yener S., Comlekci A., Ozcan MA., Kebapcilar L., Yuksel F., Yesil S. Low transforming growth factor-beta1 serum levels in idiopathic male osteoporosis. J Endocrinol Invest. 2007. 30:350–5.
64.Pierce GF., Tarpley JE., Yanagihara D., Mustoe TA., Fox GM., Thomason A. Platelet-derived growth factor (BB homodimer), transforming growth factor-beta 1, and basic fibroblast growth factor in dermal wound healing. Neovessel and matrix formation and cessation of repair. Am J Pathol. 1992. 140:1375–88.
Go to : 
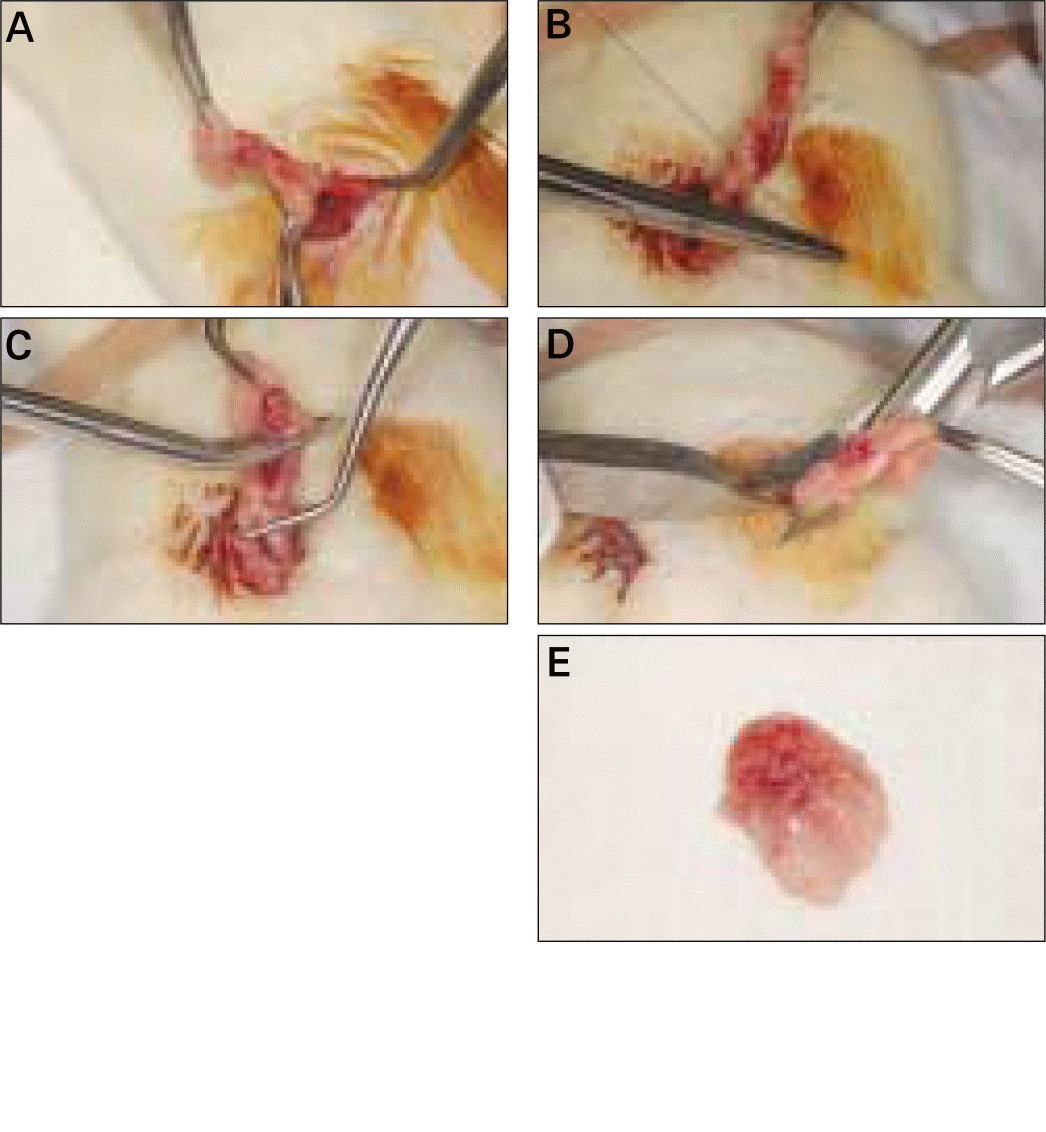 | Fig. 1.Surgical procedure of ovariec-Etomy (OVX). A, Detecting the small flower-like shaped ovary and taking it out after abdominal incision; B, Tight-suturing gently at the blood vessel to ovary; C, Excision of ovary and around tissue with scissors at the point between ovary and tight-sutured point; D, OVX accomplished in both right and left side; E, Surgically removed ovary and around fatty tissue. |
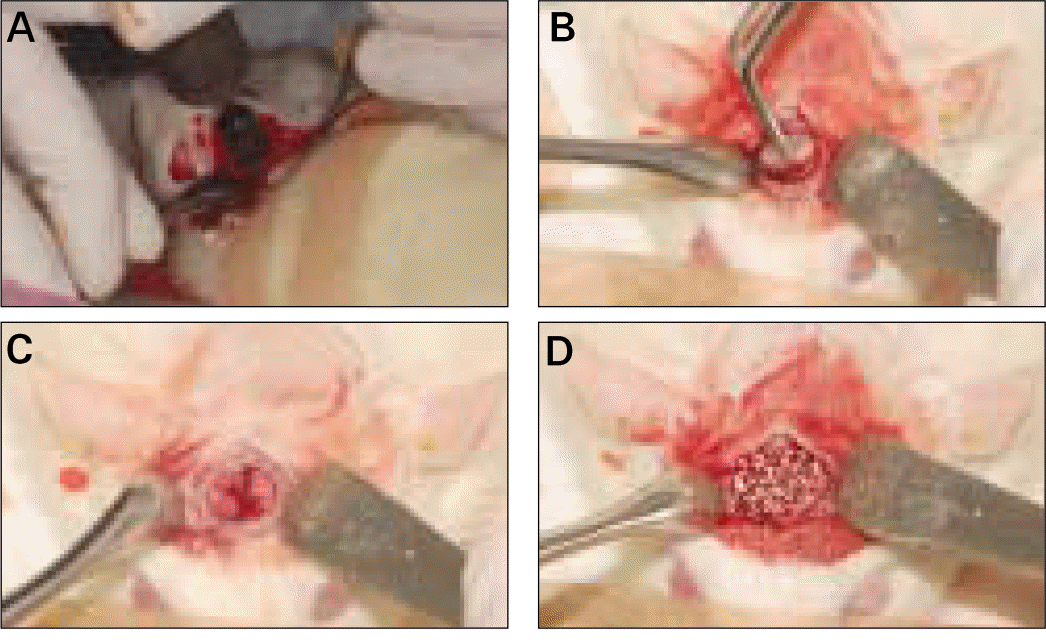 | Fig. 2.Surgical procedure of bone graft. A, Trephine drilling under saline irrigation; B, Careful removal of 8-mm-diameter calvarial bone; C, A prepared calvarial critical-sized defect (CSD); D, A CSD filled with synthetic alloplastic substitute. |
 | Fig. 3.Whole blood sample 5 ml in A and centrifuging separator (PLACONTM: Platelet Concentrator, Oscotec Inc., Cheonan, Korea) in B. |
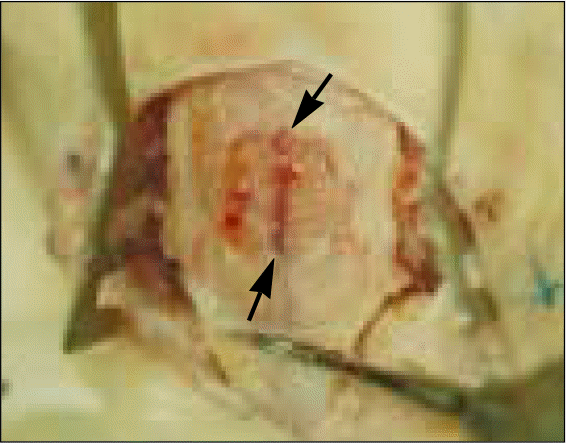 | Fig. 4.Bone sampling after sacrifice. Cutting carefully the calvarial bone more widely than outline (arrowheads) of a critical-sized defect. |
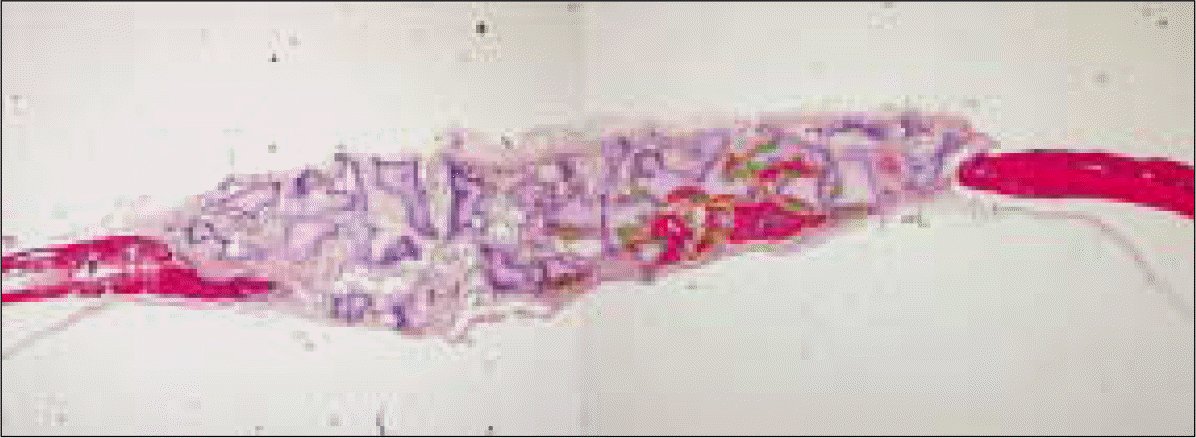 | Fig. 5.Measuring the calcified area of new bone formation. Yellow lines represent outlines of newly calcified bone after bone graft which was stained in reddish color between the non-structural synthetic alloplastic substitute (MBCPTM) particles. Green words represent serial numbers of yellow lines. |
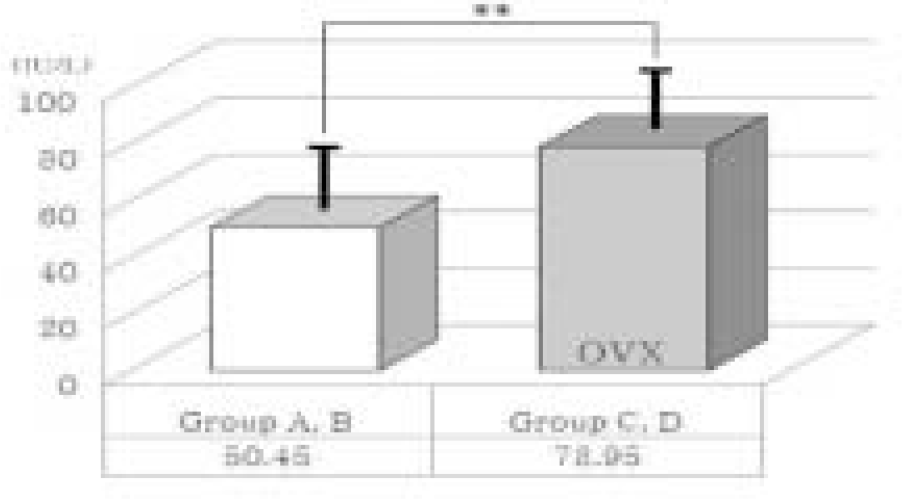 | Fig. 6.Comparison of serum alkaline phosphatase (ALP) level between normal and ovariectomized groups (OVX). Thick solid lines indicate standard deviation. ∗∗ means significant difference at P < .05 statistically. |
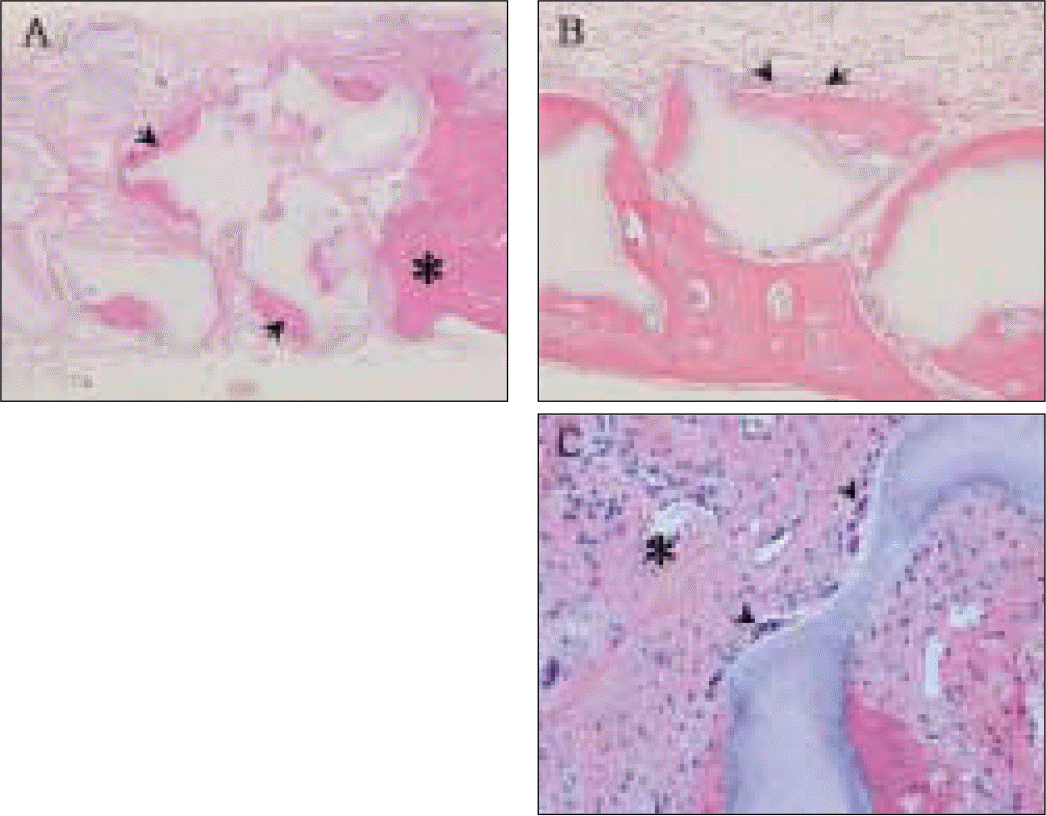 | Fig. 7.Light-microscopic view. A, Calcified area of new bone (arrowheads) mainly around outlines of synthetic alloplastic substitute particles and host calvarial bone (asterisk) at 100× magnification; B, Osteoblasts (arrowheads) lining in a row at 200× magnification; C, Multinucleated cells (arrowheads) on the surface of a synthetic alloplastic substitute particle and newly forming blood vessel (asterisk) at 400× magnification. |
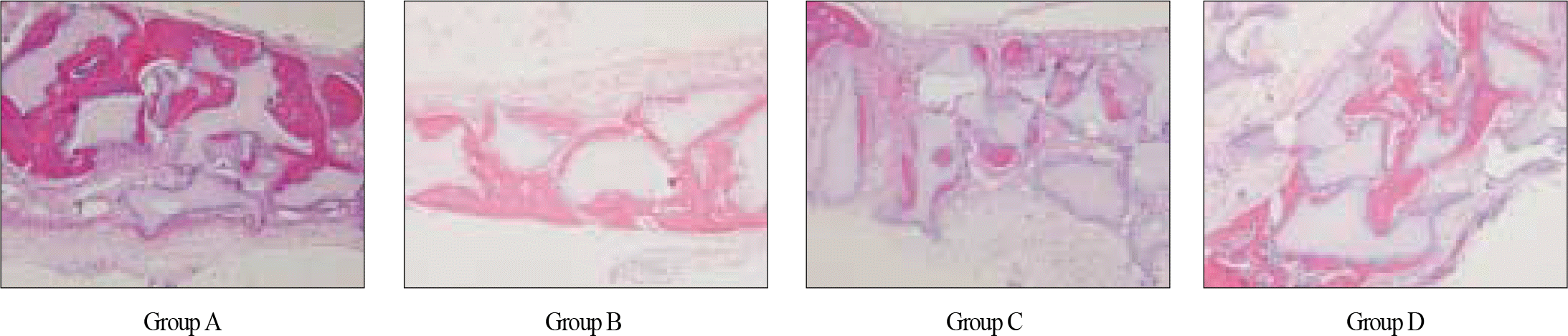 | Fig. 8.Light-microscopic view of calcified area of new bone formation in each group at 100× magnification. In group C, calcified area which was stained in reddish color is relatively smaller than that of other three groups. |
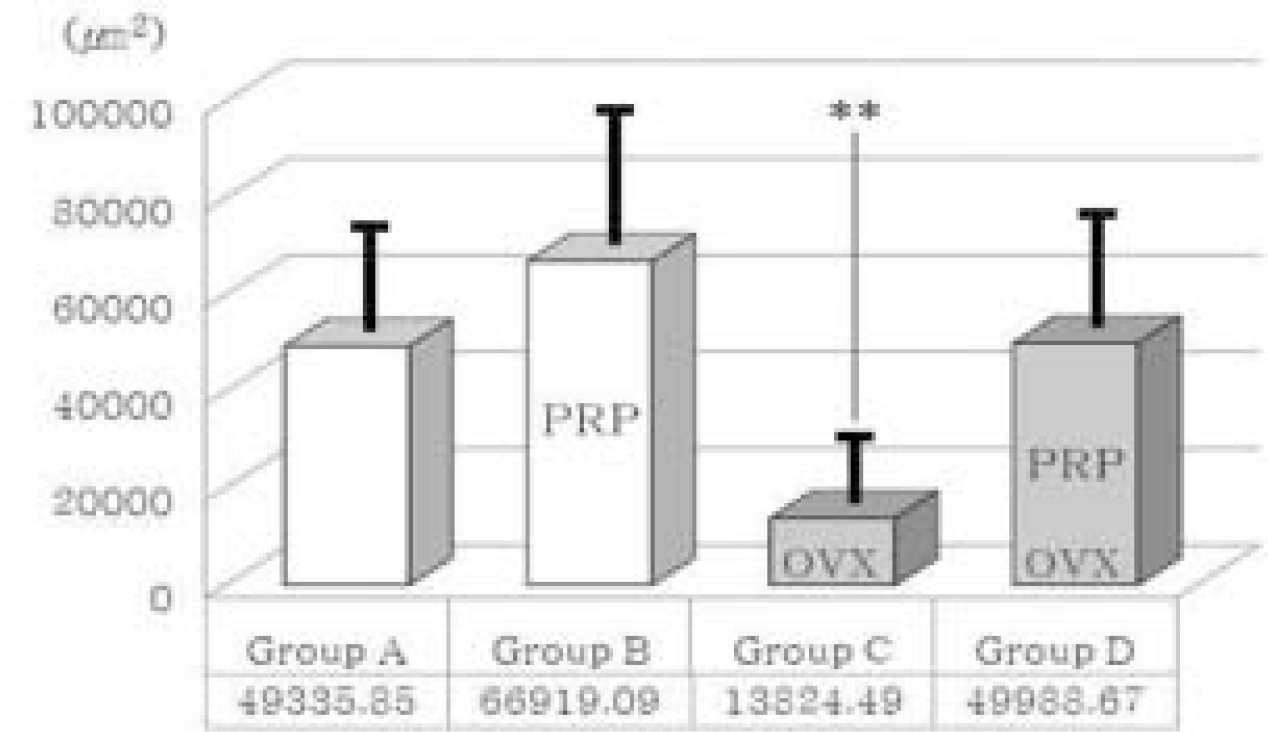 | Fig. 9.Comparison of new bone formation area. OVX; Ovariectomized group, PRP; PRP treated group. Thick solid lines indicate standard deviation. ∗∗means significant difference at P < .05 statistically. |
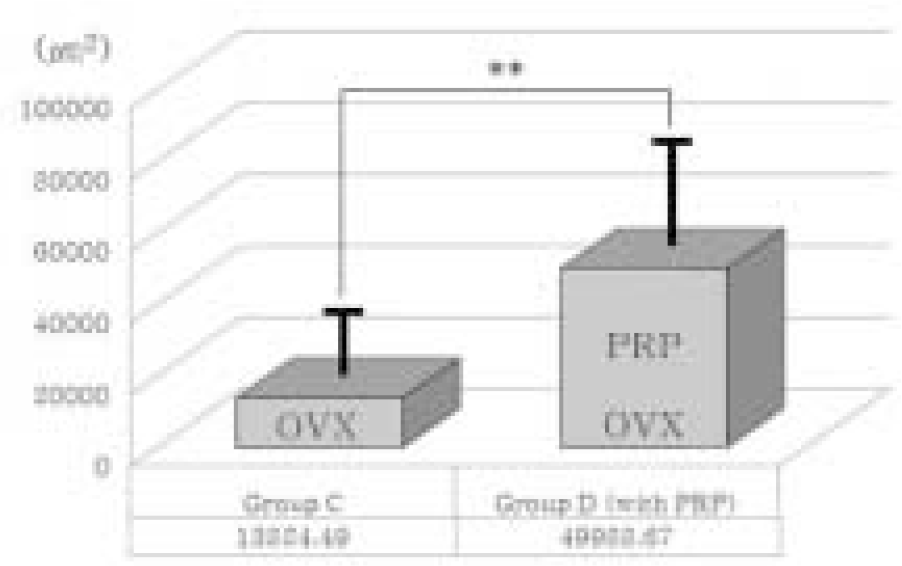 | Fig. 10.Comparison of new bone formation area between group C and group D. In ovariectomized (osteoporotic) groups (OVX), platelet-rich plasma showed a positive effect on bone regeneration significantly. PRP; PRP treated group. Thick solid lines indicate standard deviation. ∗∗means significant difference at P < .05 statistically. |
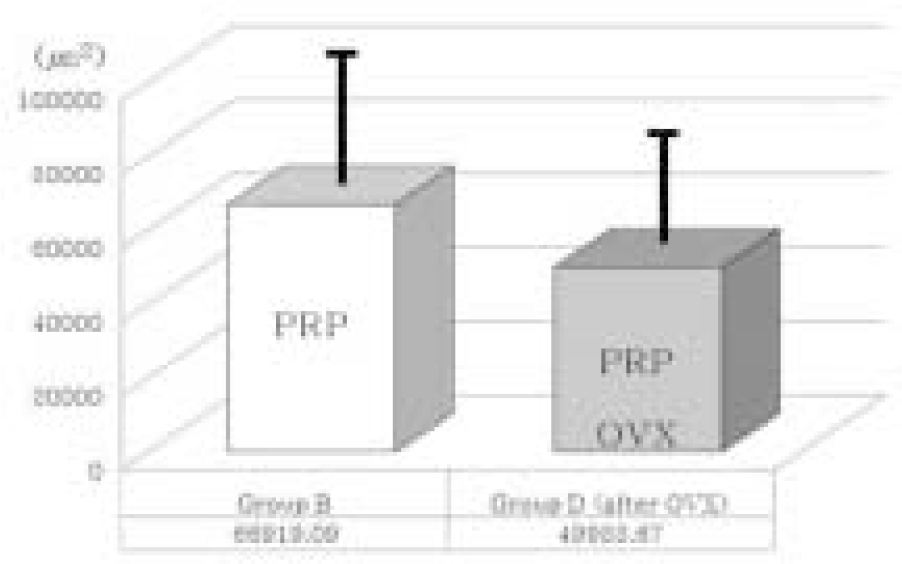 | Fig. 11.Comparison of new bone formation area between group B and group D. In PRP treated groups (PRP), ovariectomy diminished bone regeneration somewhat. However, statistically it is not significant (P > .05). OVX; Ovariectomized group. Thick solid lines indicate standard deviation. |
Table I.
Experimental groups according to OVX and PRP treatments
| Group | OVX | PRP | Graft material | N |
|---|---|---|---|---|
| A | No | No | MBCPTM | 10 |
| B | No | Yes | MBCPTM | 10 |
| C | Yes | No | MBCPTM | 10 |
| D | Yes | Yes | MBCPTM | 10 |
Table II.
Results of serum ALP level (IU/L)
| Group | N | Mean (IU/L) | SD (IU/L) |
|---|---|---|---|
| A & B (non-OVX groups) | 20 | 50.45 | 13.65 |
| C & D (OVX groups) | 20 | 78.95 | 13.64 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download