Abstract
Purpose
The objective of the present study was to histologically evaluate durability and bone regeneration capacity of new synthetic membranes in comparison to clinically available collagen membrane.
Material and methods
To the skulls of 12 rabbits, we created 4 bone defects of 6 mm in diameter on each of them. Each of defects were covered with at least one of 5 membranes: No membrane, Collagen (OssixTM), PLGA, HA-coated-PLGA and HA-PLGA/PLGA. After 4, 8, 12 weeks, we cut the skulls and dyed with H-E. And then, the histologic observation was done.
Results
In current study, the control group which did not use the membrane showed bone regeneration at 12 weeks and covered the bone defect partially. New bones were formed through the underneath of endocranium, and the upper defect was filled with connective tissues and fats. Collagen membrane (OssixTM) showed new bones after 4 weeks, and they were formed through the membrane which maintained until 12 weeks. PLGA, HA-coated-PLGA, HA-PLGA/PLGA showed bone regeneration after 4 weeks and after 8 weeks, they mostly filled defects. At 12 weeks, we could find new bones and previous bones almost look alike and also, they united well. Membranes were unnoticeable after 4 weeks and were absorbed.
Go to : 
REFERENCES
1.Ozimeric N., Bal B., Oygur T., Balos K. The effect of a collagen membrane in regenerative therapy of two-wall intrabony defects in dogs. Periodontal Clin Investing. 2000. 22:22–30.
2.Dupoirieux L., Pourquier D., Picot MC., Neves M. Comparative study of three membranes for guided bone regeneration of rat cranial defects. Int J Oral Maxillofac Surg. 2001. 30:58–62.
3.Shive MS., Anderson JM. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv Drug Deli Rev. 1997. 28:5–24.
4.Yoo HS., Lee EA., Yoon JJ., Park TG. Hyaluronic acid modified biodegradable scaffolds for cartilage tissue engineering. Biomaterials. 2005. 26:1925–133.

5.Nam YS., Yoon JJ., Park TG. A novel fabrication method for macroporous scaffolds using gas foaming salt as porogen additive. J Biomed Mater Res. 2000. 53:1–7.
6.Burg KJL., Porter S., Kellam JF. Biomaterials development for bone tissue engineering. Biomaterials. 2000. 21:2347–59.
7.Gunatillake PA., Adhikari R. Biodegradable synthetic polymers for tissue engineering. Eur Cell Mater. 2003. 20:1–16.

8.Ishaug SL., Crane GM., Miller MJ., Yasko AW., Yaszemski M., Mikos AG. Bone formation by three-dimensional stromal osteoblast culture in biodegrable polymer scaffolds. J Biomed Mater Res. 1997. 36:17–28.
9.Ochi K., Chen G., Ushida T., Gojo S., Segawa K., Tai H., Ueno K., Ohkawa H., Mori T., Yamaguchi A., Toyama Y., Hata J., Umezawa A. Use of isolated mature osteoblasts in abundance acts as desired-shaped bone generation in combination with a modified poly DL-lactic-co-glycolic acid (PLGA) -collagen sponge. J Cell Physiol. 2003. 194:45–53.
10.Kim SY., Kanamori T., Boumi Y., Wang PC., Shinbo T. Preparation of porous poly(D,L-lactide) and poly(D,L-lactide-co-glycol-ide)membranes by a phase inversion process and investigation of their morphological changes as cell culture scaffolds. J Appl Polym Sci. 2004. 92:2082–92.
12.Lee CT., Lee YD. Preparation of porous biodegradable poly(lac-tide-co-glycolide)/hyaluronic acid blend scaffolds: characterization, in vitro cells culture and degradation behaviors. J Mater Sci Mater Med. 2006. 17:1411–20.
13.West DC., Kumar S. The effect of hyaluronate and its oligosaccharides on endothelial cell proliferation and monolayer integrity. Exp Cell Res. 1989. 183:179–96.

14.Marinucci L., Lilli C., Baroni T., Becchetti E., Belcastro S., Balducci C., Locci P. In vitro comparison of bioabsorbable and non-resorbable membranes in bone regeneration. J Periodontol. 2001. 72:753–9.
15.Bergsma JE., de Bruijn WC., Rozema FR., Bos RR., Boering G. Late degradation tissue response to poly (L-lactide) bone plates and screws. Biomaterials. 1995. 16:25–31.
16.Bostman OM., Pihlajamaki HK., Partio EK., Rokkanen PU. Clinical biocompatibility and degradation of polylevolactide screws in the ankle. Clin Orthop Relat Res. 1995. 320:101–9.
17.von Arx T., Nina Broggini N., Jensen SS., Bornstein MM., Schenk RK., Buser D. Membrane durability and tissue response of different bioresorbable barrier membranes: A histologic study in the rabbit calvarium. Int J Oral Maxillofac Implants. 2005. 20:843–53.
Go to : 
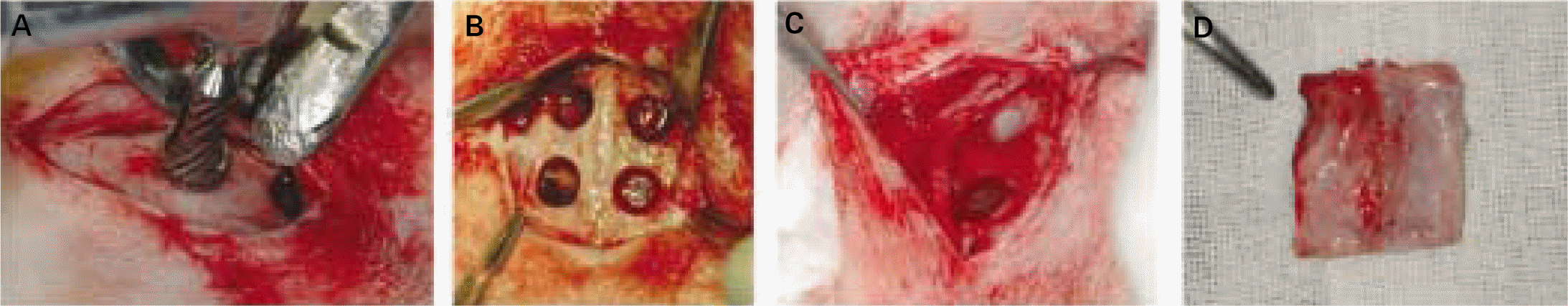 | Fig. 1.A, 6 mm diameter critical size calvarial defect was created with saline cooled trephine drill; B, 4 defects were trephined, 2 on each side of the sagittal suture. 4 defects were covered with different membranes; C, Each membrane was cut and placed individually to extend beyond the defect margins by approximately 2 mm; D, Skin incision was made, and the calvarium was removed. |
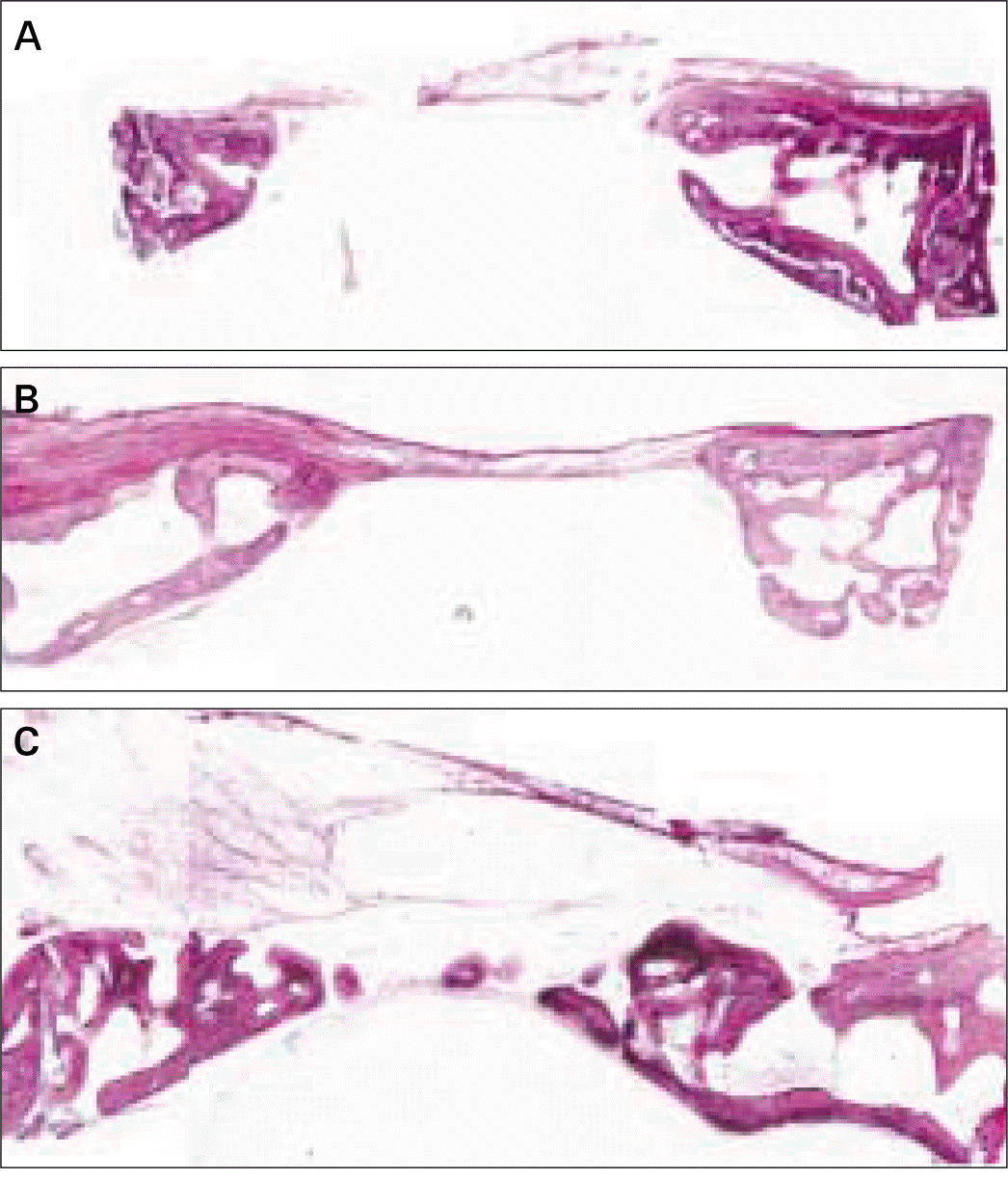 | Fig. 2.Transversal histologic sections of defects in no-membrane group. A, at 4 weeks; B, at 8 weeks; C, at 12 weeks (HE; original magnification, × 20). |
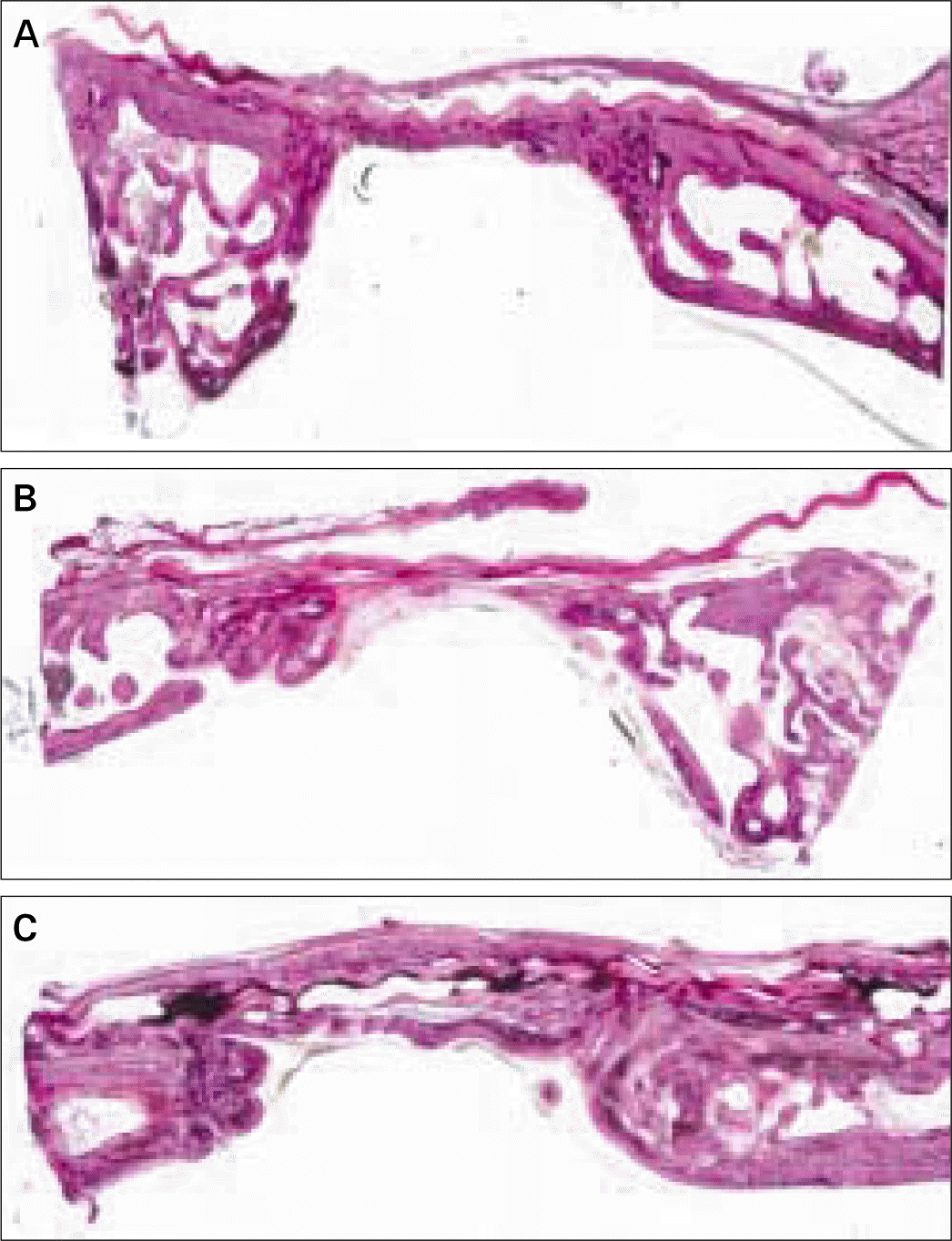 | Fig. 3.Transversal histologic sections of defects covered with OssixTM membrane. A, at 4 weeks; B, at 8 weeks; C, at 12 weeks (HE; original magnification, × 20). |
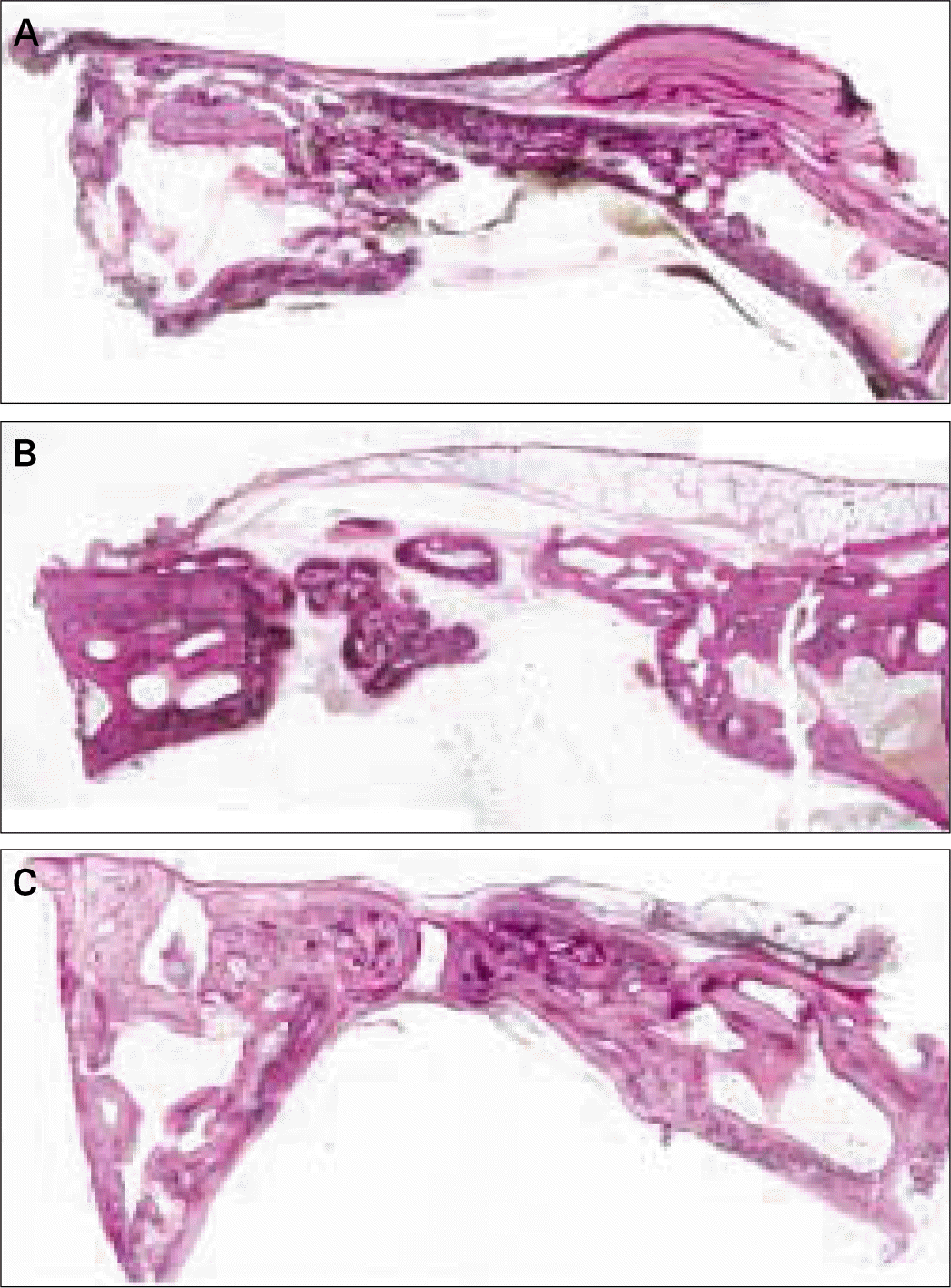
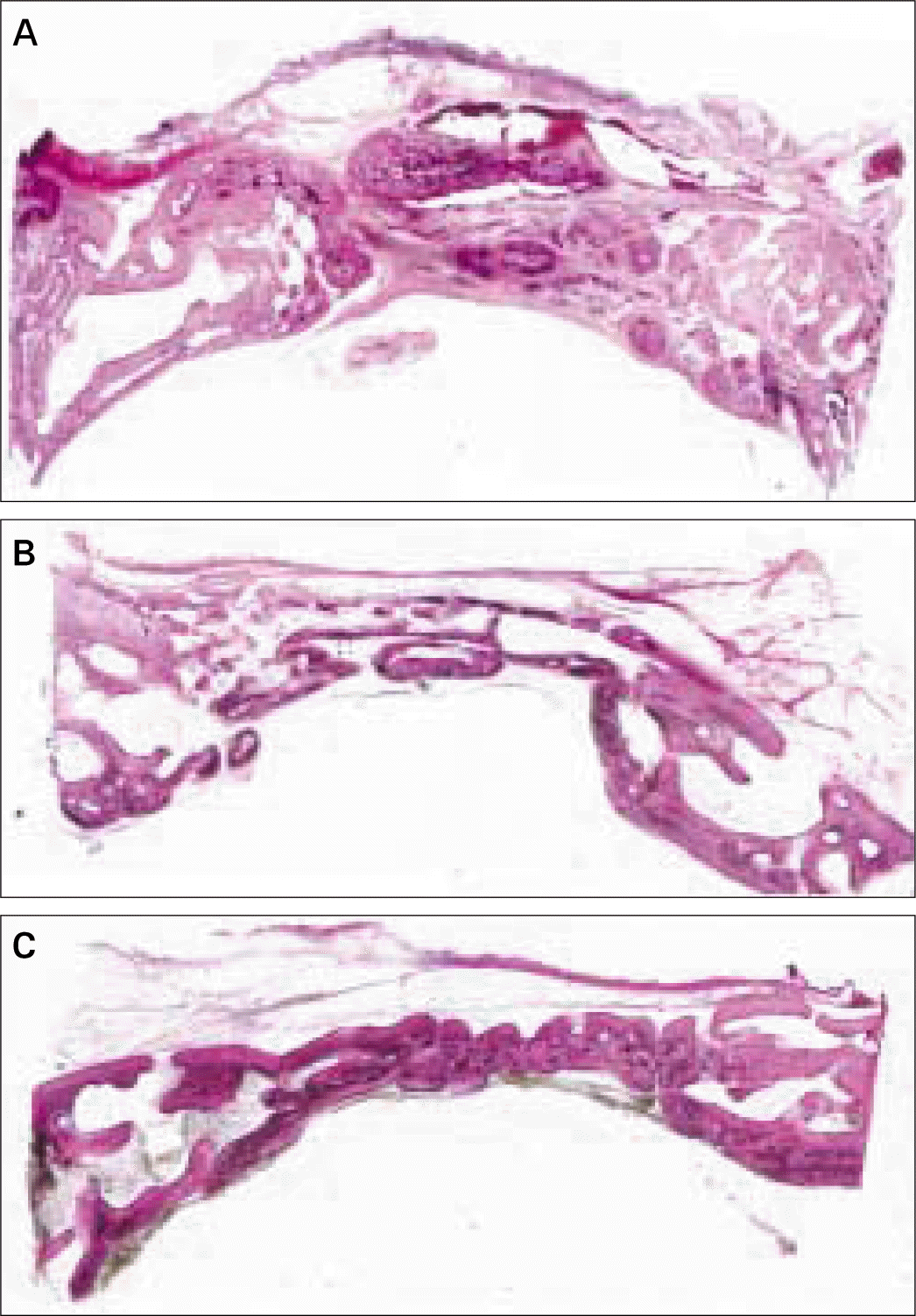 | Fig. 4.Transversal histologic sections of defects covered with PLGA membrane. A, at 4 weeks; B, at 8 weeks; C, at 12 weeks (HE; original magnification, × 20). |
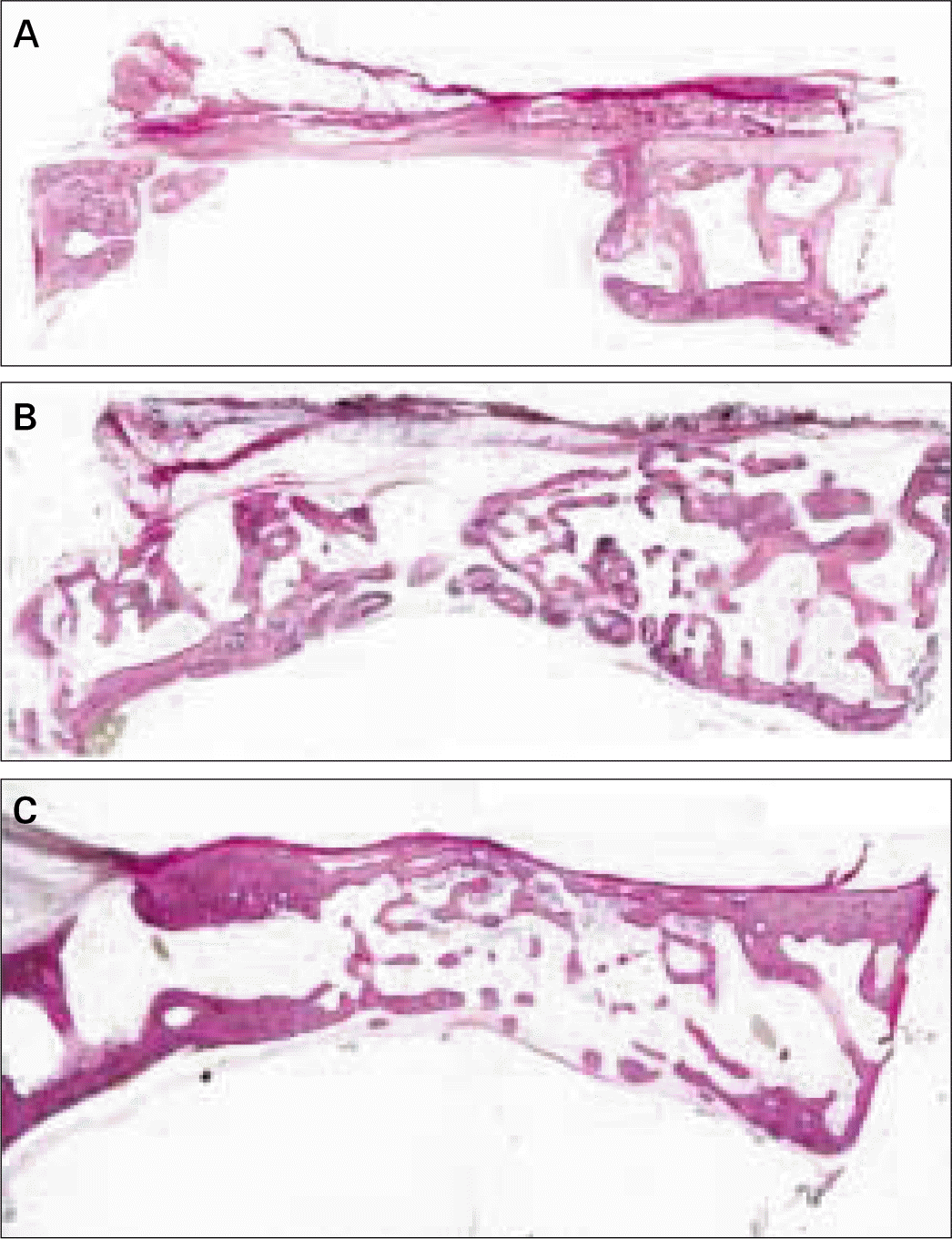 | Fig. 5.Transversal histologic sections of defects covered with HA-coated-PLGA membrane. A, at 4 weeks; B, at 8 weeks; C, at 12 weeks (HE; original magnification, × 20). |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download