Abstract
Purpose
The objective of this study was to investigate the effects of calcium phosphate coated titanium implant surface on bone response and implant stability at early stage of healing period of 3 weeks and later healing period of 6 weeks.
Material and methods
A total of 24 machined, screw-shaped implants (Dentium Co., Ltd., Seoul, Korea) which dimensions were 3.3 mm in diameter and 5.0 mm in length, were used in this research. All implants (n = 24), made of commercially pure (grade IV) titanium, were divided into 2 groups. Twelve implants (n = 12) were machined without any surface modification (control). The test implants (n = 12) were anodized and coated with thin film (150 nm) of calcium phosphate by electron-beam deposition. The implants were placed on the proximal surface of the rabbit tibiae. The bone to implant contact (BIC) ratios was evaluated after 3 and 6 weeks of implant insertion.
Results
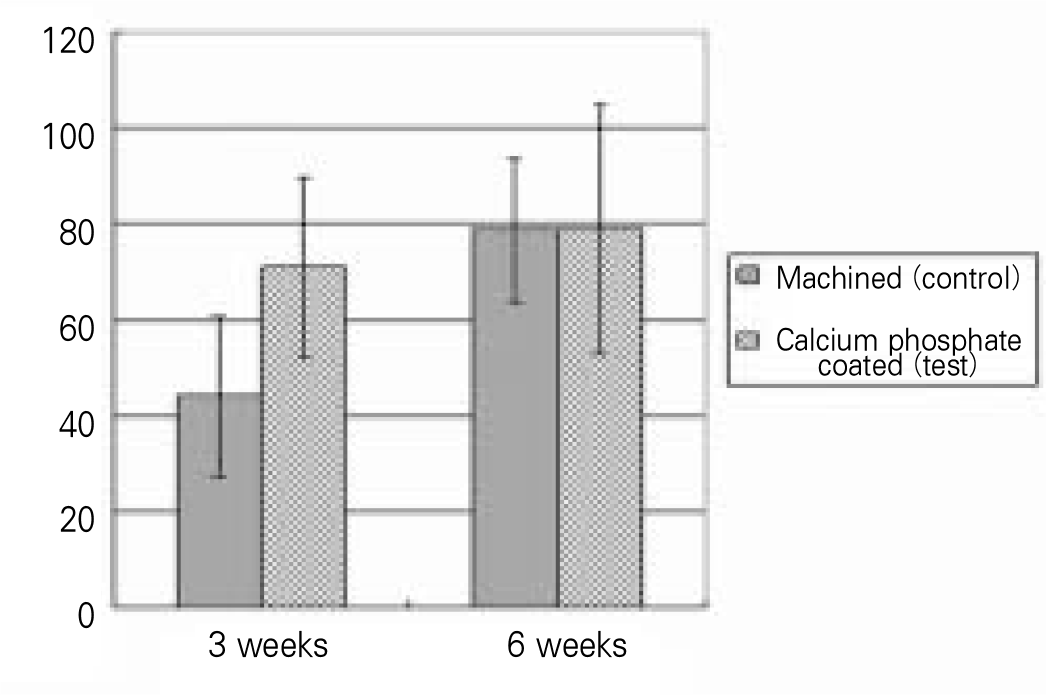
The BIC percentage of calcium phosphate coated implants (70.8 ± 18.9%) was significantly higher than that of machined implants (44.1 ± 16.5%) 3 weeks after implant insertion (P = 0.0264). However, there was no significant difference between the groups after 6 weeks of healing (P > .05).
Conclusion
The histomorphometric evaluation of implant surface revealed that: 1. After 3 weeks early healing period, bone to implant contact (BIC) percentage of calcium phosphate coated implants (70.8%) was much greater than that of surface untreated machined implants (44.1%) with P = 0.0264. 2. After 6 weeks healing period, however, BIC percentage of calcium phosphate coated implants group (79.0%) was similar to the machined only implant group (78.6%). There was no statistical difference between two groups (P = 0.8074). 3. We found the significant deference between the control group and experimental group during the early healing period of 3 weeks. But no statistical difference was found between two groups during the later of 6 weeks. (J Korean Acad Prosthodont 2010;48:122-7)
Go to : 
REFERENCES
1.Eisenbarth E., Velten D., Schenk-Meuser K., Linez P., Biehl V., Duschner H., Breme J., Hildebrand H. Interactions between cells and titanium surfaces. Biomol Eng. 2002. 19:243–9.

2.Bra � nemark PI., Breine U., Adell R., Hansson BO., Lindstro ¨m J., Ohlsson A. Intra-osseous anchorage of dental prostheses. Scand J Plast Reconstr Surg. 1969. 3:81–100.
3.Albrektsson T., Bra � nemark PI., Hansson HA., Lindstro ¨m J. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop Scand. 1981. 52:155–70.
4.Wennerberg A., Albrektsson T., Ulrich H., Krol JJ. An optical three-dimensional technique for topographical descriptions of surgical implants. J Biomed Eng. 1992. 14:412–8.

5.Albrektsson T., Sennerby L., Wennerberg A. State of the art of oral implants. Periodontol 2000. 2008. 47:15–26.
6.Wennerberg A., Albreksson T., Andersson B. Bone tissue response to commercially pure titanium implants blasted with fine and coarse particles of aluminum oxide. Int J Oral Maxillofac Implants. 1996. 11:38–45.
7.Le Gue′hennec L., Soueidan A., Layrolle P., Amouriq Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent Mater. 2007. 23:844–54.
8.Vanzillotta PS., Sader MS., Bastos IN., Soares Gde A. Improvement of in vitro titanium bioactivity by three different surface treatments. Dent Mater. 2006. 22:275–82.
9.Daculsi G., Laboux O., Malard O., Weiss P. Current state of the art of biphasic calcium phosphate bioceramics. J Mater Sci Mater Med. 2003. 14:195–200.
11.Lee EJ., Lee SH., Kim HW., Kong YM., Kim HE. Fluoridated apatite coatings on titanium obtained by electron-beam deposition. Biomaterials. 2005. 26:3843–51.

12.Donath K., Breuner G. A method for the study of undecalcified bones and teeth with attached soft tissues. The Sa ¨ge-Schliff (sawing and grinding) technique. J Oral Pathol. 1982. 11:318–26.
13.Wagner WC. A Brief Introduction to advanced surface modification technologies. J Oral Implantol. 1992. 18:231–5.
14.Barre`re F., van der Valk CM., Meijer G., Dalmeijer RA., de Groot K., Layrolle P. Osteointegration of biomimetic apatite coating applied onto dense and porous metal implants in femurs of goats. J Biomed Mater Res B Appl Biomater. 2003. 67:655–65.
15.Lee JJ., Rouhfar L., Beirne OR. Survival of hydroxyapatite-coated implants: a meta-analytic review. J Oral Maxillofac Surg. 2000. 58:1372–9.

16.Roberts WE., Smith RK., Zilberman Y., Mozsary PG., Smith RS. Osseous adaptation to continuous loading of rigid endosseous implants. Am J Orthod. 1984. 86:95–111.

Go to : 
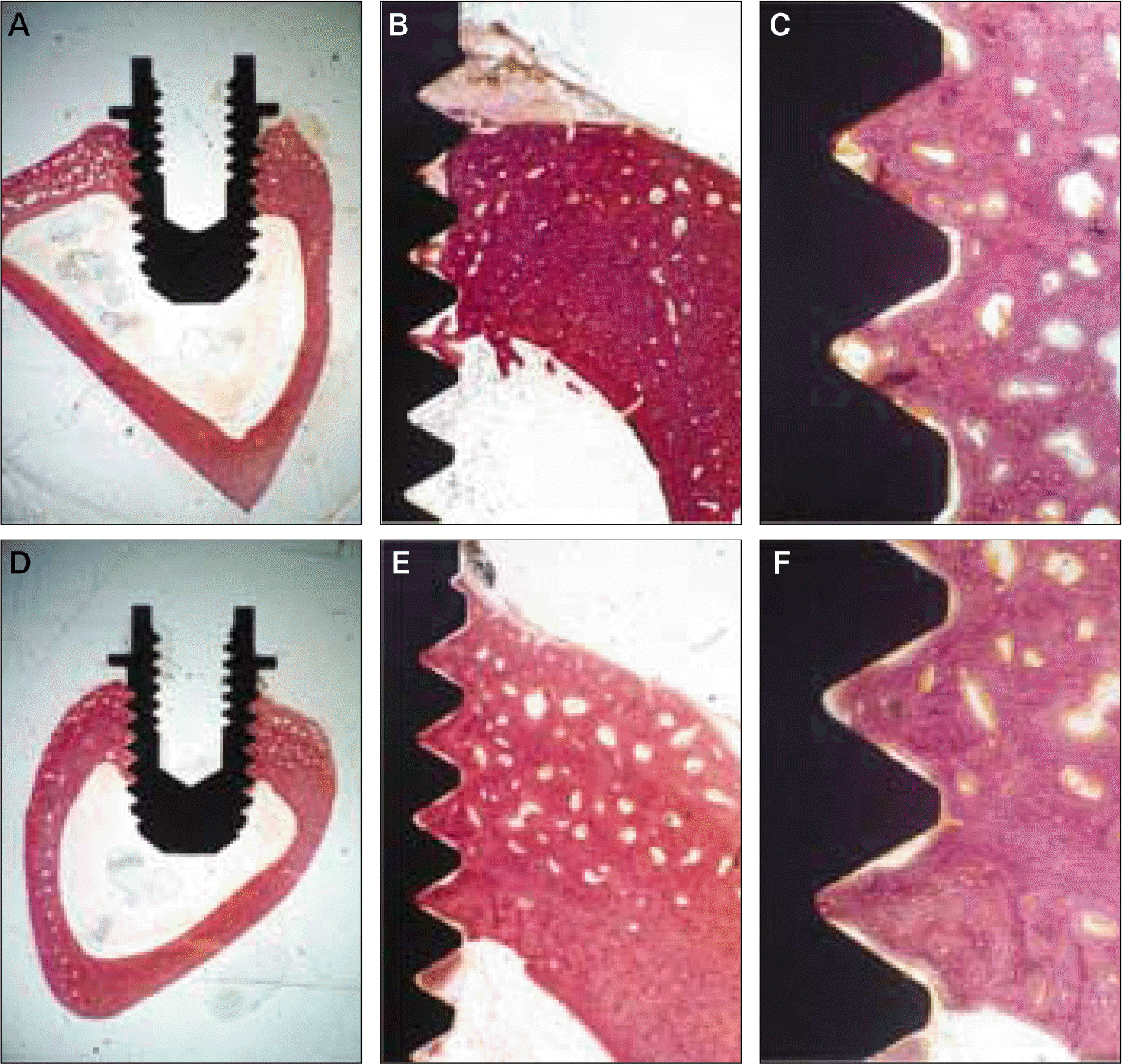
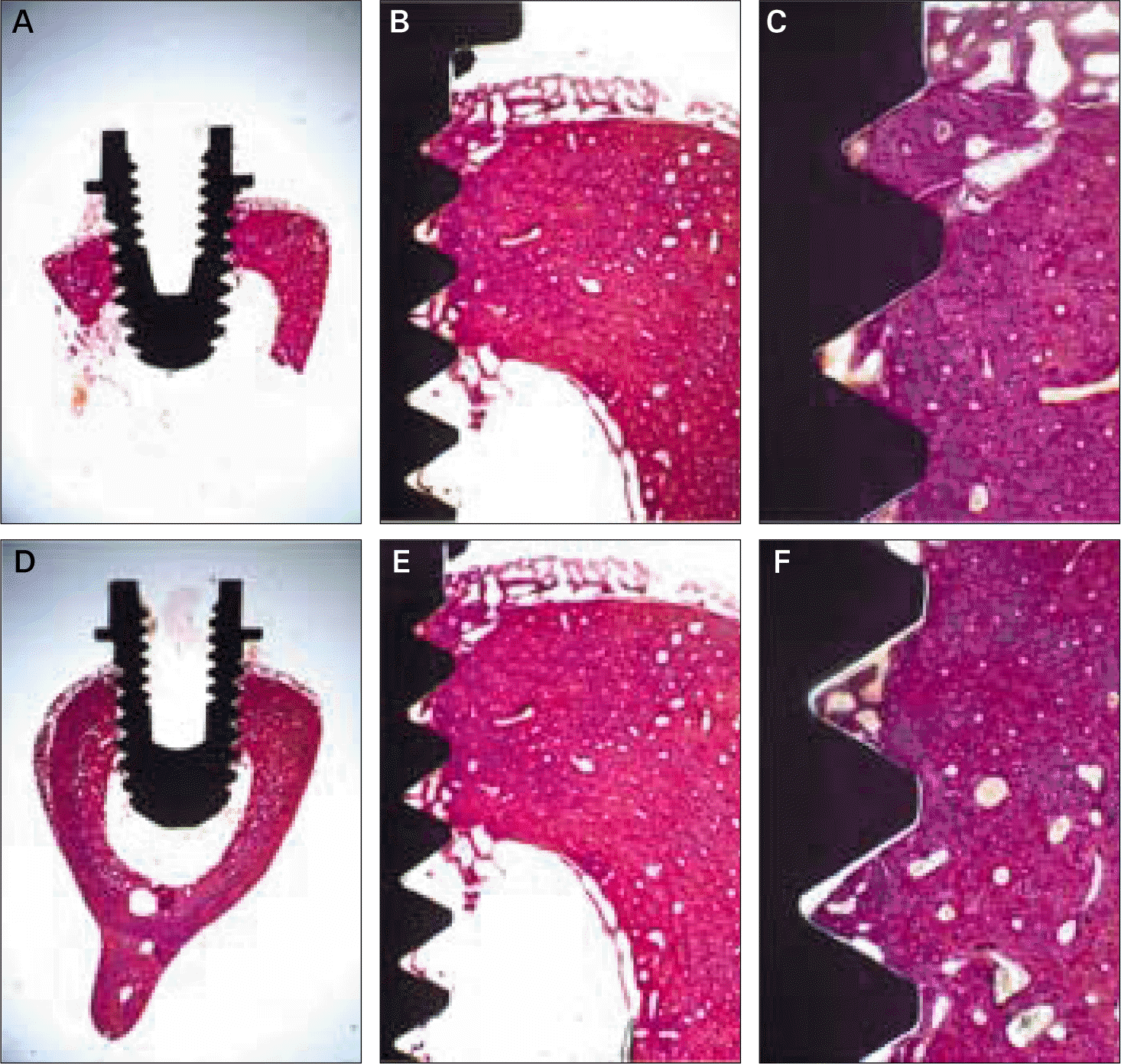 | Fig. 2.Histologic views at three magnifications (× 10, × 40, and × 100) of (A)-(C) machined and (D)-(F) calcium phosphate coated implants after 3 weeks of healing. Much woven bone was observed to contact the dental implant. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download