Abstract
Statement of problem
Many studies have been conducted to improve the primary stability of implants by providing bioactive surfaces via surface treatments. Increase of surface roughness may increase osteoblast activity and promote stronger bonding between bone and implant surface and it has been reported that bioactive surface or titanium can be obtained through alkali and heat treatment.
Purpose
The purpose of this study was to evaluate the stability of alkali and heat treated implants via histomorphometric analysis.
Material and methods
Specimens were divided into three groups; group 1 was the control group with machined surface, the other groups were treated for 24 hours in 5 M NaOH solution and heat treated for 1 hour at 600℃ in the atmosphere (group 2) and vacuum (group 3) conditions respectively. Surface characteristics were analyzed and fixtures were implanted into rabbits. The specimens were histologically and histomorphometrically compared according to healing periods and change in bone composition were analyzed with EPMA (Electron Probe Micro Analyzer).
Results
1. Groups treated with alkali and heat showed increase of oxidization layer and Na ions. Groups 2 which was heat treated in atmosphere showed significant increase of surface roughness (P < .05). 2. Histomorphometric analysis showed significant increase in BIC (bone to implant contact) according to increase in healing period and there was significant increases in groups 2 and 3 (P < .05). 3. BA(bone area) ratio showed similar results as contact ratio, but according to statistical analysis there was significant increase according to increase in healing period in group 2 only (P < .05). 4. EPMA analysis revealed no difference in gradation of bone composition of K, P, Ca, Ti in surrounding bone of implants according to healing periods but groups 2 and 3 showed increase of Ca and P in the initial stages.
Go to : 
REFERENCES
1.Bra � nemark P., Zarb G., Albrektsson T. Tissue integrated prostheses: Osseointegration in clinical dentistry. Chicago: Quintessence;Publishing Co.1985. p. p221–9.
2.Albrektsson T., Bra � nemark PI., Hansson HA., Lindstro ¨m J. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop Scand. 1981. 52:155–70.
3.Buser D., Schenk RK., Steinemann S., Fiorellini JP., Fox CH., Stich H. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J Biomed Mater Res. 1991. 25:889–902.

4.Choi JW., Kim KN., Heo SJ., Chang IT., Han JH., Baik HK., Choi YC., Wennerberg A. The effects of various surface treatment methods on the osseointegration. J Korean Acad Prosthodont. 2001. 39:71–83.
5.Kang BS., Cho IH. A histomorphometric and stability of two kinds of implants with different surface roughness. J Korean Acad Oral Maxillofac Implants. 2001. 5:42–69.
6.Larsson C., Thomsen P., Lausmaa J., Rodahl M., Kasemo B., Ericson LE. Bone response to surface modified titanium implants: studies on electropolished implants with different oxide thicknesses and morphology. Biomaterials. 1994. 15:1062–74.

7.Wennerberg A., Albrektsson T. Suggested guidelines for the topographic evaluation of implant surfaces. Int J Oral Maxillofac Implants. 2000. 15:331–44.
8.Wong M., Eulenberger J., Schenk R., Hunziker E. Effect of surface topology on the osseointegration of implant materials in trabecular bone. J Biomed Mater Res. 1995. 29:1567–75.

9.Kasemo B., Lausmaa J. Biomaterial and implant surfaces: a surface science approach. Int J Oral Maxillofac Implants. 1988. 3:247–59.
10.Wennerberg A., Albrektsson T., Lausmaa J. Torque and histomorphometric evaluation of c.p. titanium screws blasted with 25- and 75-microns-sized particles of Al2O3. J Biomed Mater Res. 1996. 30:251–60.
11.Park KH., Chang IT. Osseointegration of anodized titanium implants. J Korean Acad Prosthodont. 2004. 42:267–77.
12.Eliades T. Passive film growth on titanium alloys: physicochemical and biologic considerations. Int J Oral Maxillofac Implants. 1997. 12:621–7.
13.Pan J., Thierry D., Leygraf C. Electrochemical impedance spectroscopy study of the passive oxide film on titanium for implant application. Electrochim Acta. 1996. 41:1143–53.

14.Lautenschlager EP., Monaghan P. Titanium and titanium alloys as dental materials. Int Dent J. 1993. 43:245–53.
15.Brauner H. Corrosion resistance and biocompatibility of physical vapour deposition coatings for dental applications. Surf Coat Technol. 1993. 62:618–25.

16.Kieswetter K., Schwartz Z., Dean DD., Boyan BD. The role of implant surface characteristics in the healing of bone. Crit Rev Oral Biol Med. 1996. 7:329–45.

17.Peutzfeldt A., Asmussen E. Distortion of alloy by sandblasting. Am J Dent. 1996. 9:65–6.
18.Pouilleau J., Devilliers D., Garrido F., Durand-Vidal S., Mahe E. Structure and composition of passive titanium oxide film. Mater Sci Eng. 1997. 47:235–43.
19.Sul YT., Byon ES., Jeong Y. Biomechanical measurements of calcium-incorporated oxidized implants in rabbit bone: effect of calcium surface chemistry of a novel implant. Clin Implant Dent Relat Res. 2004. 6:101–10.

20.Sul YT., Johansson C., Albrektsson T. Which surface properties enhance bone response to implants? Comparison of oxidized magnesium, TiUnite, and Osseotite implant surfaces. Int J Prosthodont. 2006. 19:319–28.
21.Sul YT., Johansson CB., Jeong Y., Ro ¨ser K., Wennerberg A., Albrektsson T. Oxidized implants and their influence on the bone response. J Mater Sci Mater Med. 2001. 12:1025–31.
22.Sul YT., Johansson C., Wennerberg A., Cho LR., Chang BS., Albrektsson T. Optimum surface properties of oxidized implants for reinforcement of osseointegration: surface chemistry, oxide thickness, porosity, roughness, and crystal structure. Int J Oral Maxillofac Implants. 2005. 20:349–59.
23.Babbush CA., Kent JN., Misiek DJ. Titanium plasma-sprayed (TPS) screw implants for the reconstruction of the edentulous mandible. J Oral Maxillofac Surg. 1986. 44:274–82.

24.Gotfredsen K., Wennerberg A., Johansson C., Skovgaard LT., Hj � rting-Hansen E. Anchorage of TiO2-blasted, HA-coated, and machined implants: an experimental study with rabbits. J Biomed Mater Res. 1995. 29:1223–31.
25.Sanz A., Oyarzu ´ n A., Farias D., Diaz I. Experimental study of bone response to a new surface treatment of endosseous titanium implants. Implant Dent. 2001. 10:126–31.
26.Marinho VC., Celletti R., Bracchetti G., Petrone G., Minkin C., Piattelli A. Sandblasted and acid-etched dental implants: a histologic study in rats. Int J Oral Maxillofac Implants. 2003. 18:75–81.
27.Ishizawa H., Ogino M. Characterization of thin hydroxyapatite layers formed on anodic titanium oxide films containing Ca and P by hydrothermal treatment. J Biomed Mater Res. 1995. 29:1071–9.

28.Lee JM., Kim YS., Kim CW., Jang KS., Lim YJ. A study on the responses of osteoblasts to various surface-treated titanium. J Korean Acad Prosthodont. 2003. 42:307–19.
29.Darvell BW., Samman N., Luk WK., Clark RK., Tideman H. Contamination of titanium castings by aluminium oxide blasting. J Dent. 1995. 23:319–22.

30.Kim HM., Miyaji F., Kokubo T., Nakamura T. Effect of heat treatment on apatite-forming ability of Ti metal induced by alkali treatment. J Mater Sci Mater Med. 1997. 8:341–7.
31.Kim HW., Kim HE., Salih V., Knowles JC. Sol-gel-modified titanium with hydroxyapatite thin films and effect on osteoblast-like cell responses. J Biomed Mater Res A. 2005. 74:294–305.

32.Kokubo T., Kim HM., Kawashita M., Nakamura T. Bioactive metals: preparation and properties. J Mater Sci Mater Med. 2004. 15:99–107.
33.Kokubo T., Miyaji F., Kim HM., Nakamura T. Spontaneous formation of bonelike apatite layer on chemically treated titanium metals. J Am Ceram Soc. 1996. 79:1127–9.

34.Wang CX., Wang M., Zhou X. Nucleation and growth of apatite on chemically treated titanium alloy: an electrochemical impedance spectroscopy study. Biomaterials. 2003. 24:3069–77.

35.Song YS., Cho IH. Surface characteristics and stability of implants treated with alkali and heat. J Korean Acad Prosthodont. 2008. 46:490–9.

36.Donath K., Breuner G. A method for the study of undecal-cified bones and teeth with attached soft tissues. The Sa ¨ge-Schliff (sawing and grinding) technique. J Oral Pathol. 1982. 11:318–26.
37.Johansson CB., Albrektsson T. A removal torque and histomorphometric study of commercially pure niobium and titanium implants in rabbit bone. Clin Oral Implants Res. 1991. 2:24–9.

38.Lim JB., Cho IH. A study on the surface characteristics and stability of implants treated with anodic oxidation and fluo-rideincorporation. J Korean Acad Stomatognathic Func Occl. 2006. 22:349–64.
40.Chosa N., Taira M., Saitoh S., Sato N., Araki Y. Characterization of apatite formed on alkaline-heat-treated Ti. J Dent Res. 2004. 83:465–9.

41.Nishiguchi S., Nakamura T., Kobayashi M., Kim HM., Miyaji F., Kokubo T. The effect of heat treatment on bone-bonding ability of alkali-treated titanium. Biomaterials. 1999. 20:491–500.

42.Jona ´ sova ´L., Mu ¨ller FA., Helebrant A., Strnad J., Greil P. Hydroxyapatite formation on alkali-treated titanium with different content of Na+ in the surface layer. Biomaterials. 2002. 23:3095–101.

43.Maitz MF., Poon RW., Liu XY., Pham MT., Chu PK. Bioactivity of titanium following sodium plasma immersion ion implantation and deposition. Biomaterials. 2005. 26:5465–73.

44.Sennerby L., Thomsen P., Ericson L. Early tissue response to titanium implants inserted in rabbit cortical bone, Part 1: Light microscopic observations. J Mater in Med. 1993. 4:240–50.
45.Sennerby L., Thomsen P., Ericson L. Early tissue response to titanium implants inserted in rabbit cortical bone, Part 2: Ultrastuctural observations. J Mater in Med. 1993. 4:494–502.
46.Tzaphlidou M, Speller R, Royle G, J Griffiths. High resolution Ca/P maps of bone architecture. Research Highlights. 2002-2003. 76–9.
Go to : 
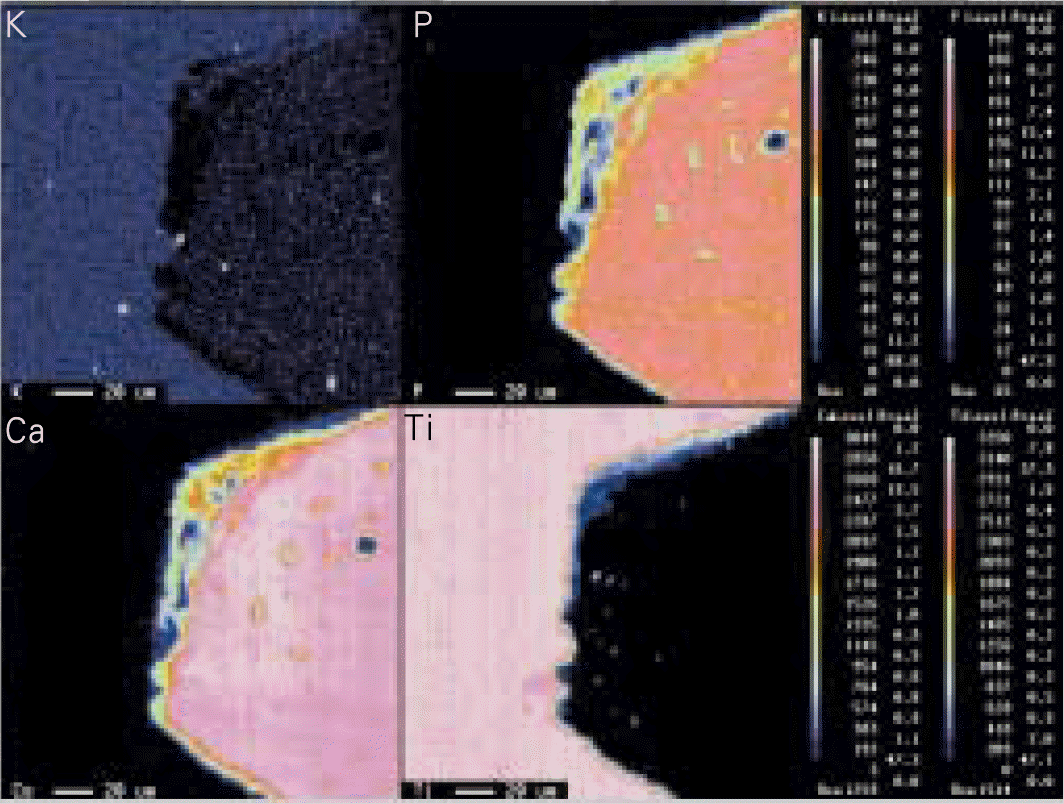 | Fig. 1.Example of EPMA mapping result. I: implant, B: bone (K: upper left, P: upper right, Ca: lower left, Ti: lower right). |
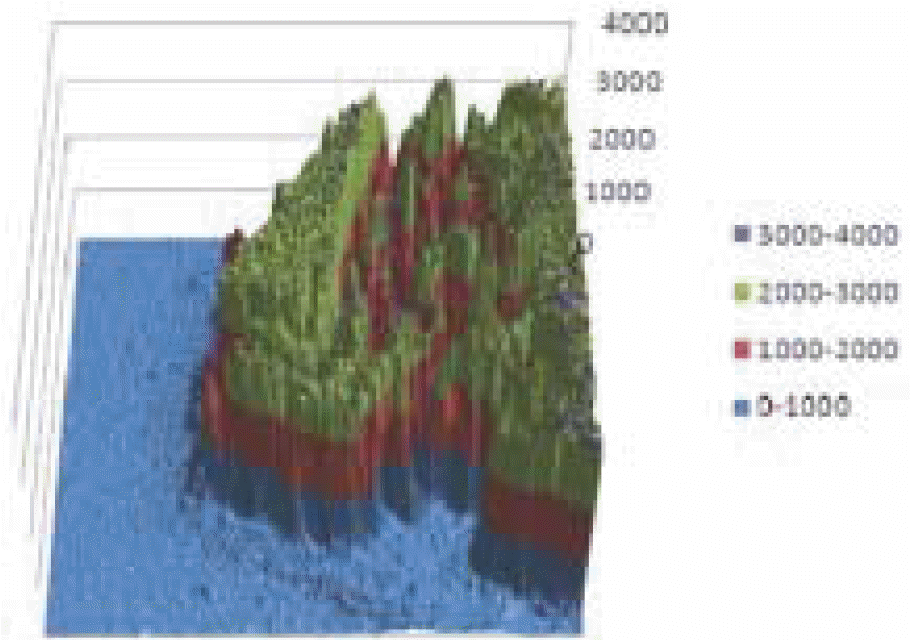 | Fig 2.3D reconstruction result of EPMA (Electron Probe Micro Analyzer) data of Ca at 6 week. |
 | Fig. 4.FESEM (Field Emition Scanning Electron Microscope) findings of implant surface. Group 1: Machined, Group 2: Treated with alkali and heat in atmosphere, Group 3: Treated with alkali and heat in vacuum. |
 | Fig. 5.3D (Dimension) Result of AFM (Atomic Force Microscope) scanning. Group 1: Machined, Group 2: Treated with alkali and heat in atmosphere, Group 3: Treated with alkali and heat in vacuum. |
 | Fig. 6.BIC (Bone to Implant Contact) and BA (Bone Area) measuring at 2 weeks. Group 1: Machined, Group 2: Treated with alkali and heat in atmosphere, Group 3: Treated with alkali and heat in vacuum. |
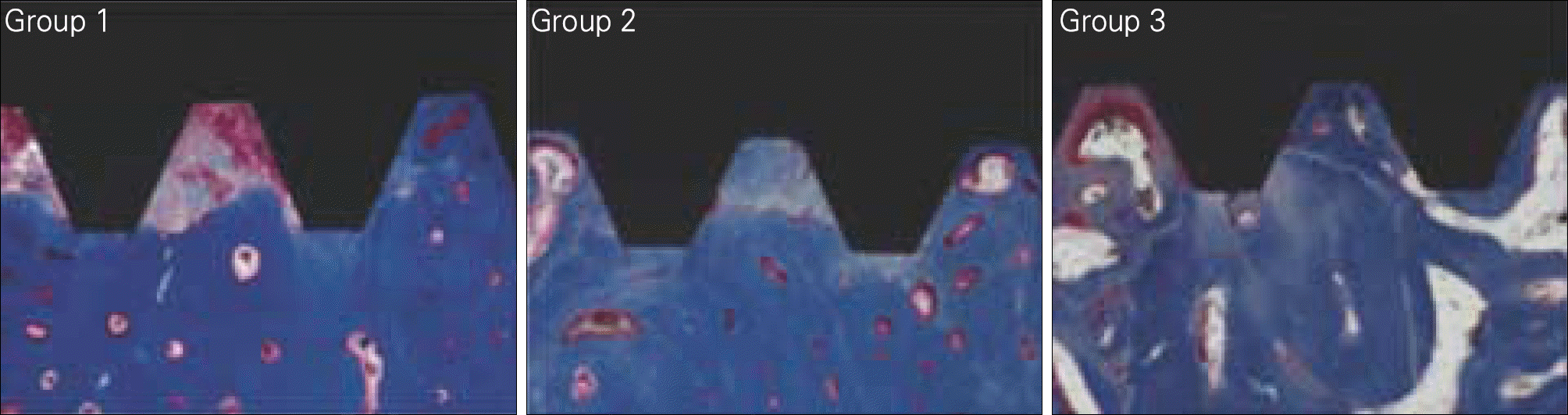 | Fig. 7.BIC (Bone to Implant Contact) and BA (Bone Area) measuring at 4 weeks. Group 1: Machined, Group 2: Treated with alkali and heat in atmosphere, Group 3: Treated with alkali and heat in vacuum. |
 | Fig. 8.BIC (Bone to Implant Contact) and BA (Bone Area) measuring at 6 weeks. Group 1: Machined, Group 2: Treated with alkali and heat in atmosphere, Group 3: Treated with alkali and heat in vacuum. |
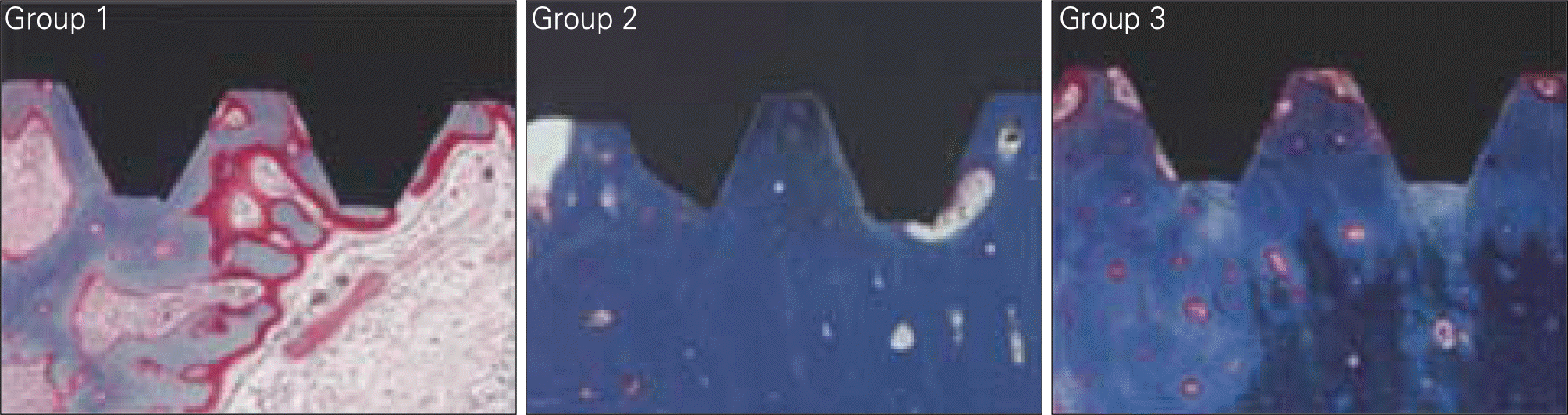 | Fig. 9.BIC (Bone to Implant Contact) and BA (Bone Area) measuring at 8 weeks. Group 1: Machined, Group 2: Treated with alkali and heat in atmosphere, Group 3: Treated with alkali and heat in vacuum. |
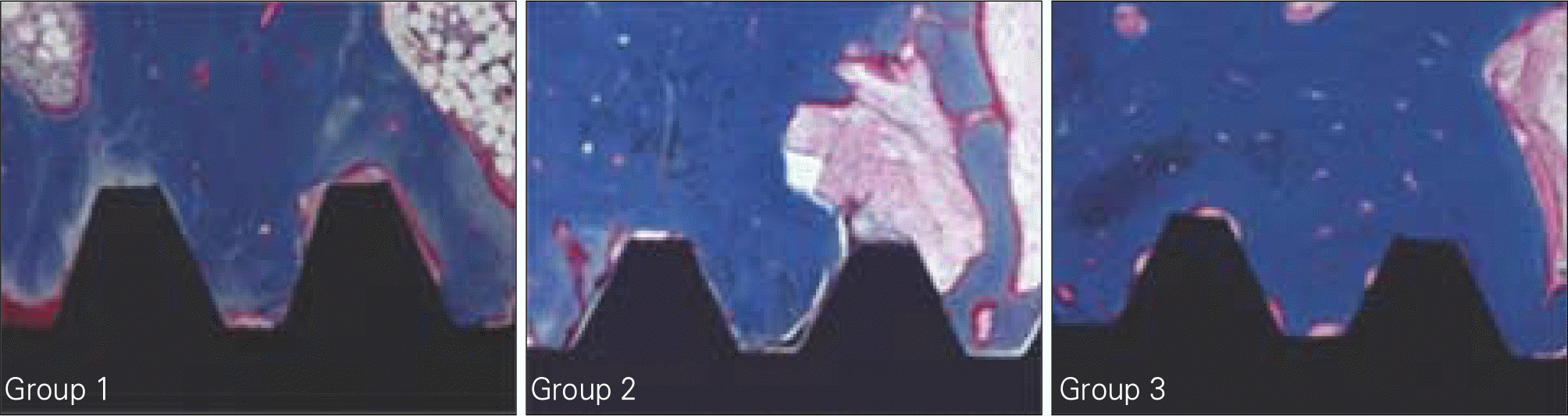 | Fig. 10.BIC (Bone to Implant Contact) and BA (Bone Area) measuring at 12 weeks. Group 1: Machined, Group 2: Treated with alkali and heat in atmosphere, Group 3: Treated with alkali and heat in vacuum. |
Table I.
Surface treatment of each groups
| Group | Preparation | N |
|---|---|---|
| 1 | Machined | 40 |
| 2 | 5M Alkali (24 H) + 600℃ heat treatment (1 H) in atmosphere | 40 |
| 3 | 5M Alkali (24H) + 600℃ heat treatment (1 H) in vacuum | 40 |
Table II.
Surface roughness of implant measured with AFM unit: μ m
| Surface | Mean | SD | |
|---|---|---|---|
| Group 1 | Sa Sq | 0.10∗ 0.13∗∗ | 0.009 0.013 |
| Group 2 | Sa Sq | 0.24∗ 0.30∗∗ | 0.080 0.094 |
| Group 3 | Sa Sq | 0.17 0.22 | 0.022 0.031 |
Table III.
The mean and standard deviation of bone to implant contact
Table IV.
Results of multiple range test for BIC ratio of Group 2 (Duncan test)
| 2 week (0.59 ± 0.36) | 4 week (0.86 ± 0.22) | 6 week (0.89 ± 0.11) | 8 week (0.75 ± 0.24) | 12 week (0.85 ± 0.18) |
|---|---|---|---|---|
| 2 week | ∗ | ∗ | ∗ | ∗ |
| 4 week | ||||
| 6 week | ||||
| 8 week | ||||
| 12 week |
Table V.
Results of multiple range test for BIC ratio of Group 3 (Duncan test)
| 2 week (0.46 ± 0.28) | 4 week (0.80 ± 0.18) | 6 week (0.65 ± 0.15) | 8 week (0.71 ± 0.21) | 12 week (0.84 ± 0.15) |
|---|---|---|---|---|
| 2 week | ∗ | ∗ | ∗ | |
| 4 week | ∗ | |||
| 6 week | ||||
| 8 week | ||||
| 12 week |
Table VI.
The mean and standard deviation of bone area
Table VII.
Results of multiple range test for BA ratio of Group 2 (Duncan test)
| 2 week (0.64 ± 0.19) | 4 week (0.66 ± 0.20) | 6 week (0.81 ± 0.14) | 8 week (0.78 ± 0.14) | 12 week (0.82 ± 0.16) |
|---|---|---|---|---|
| 2 week | ∗ | ∗ | ||
| 4 week | ∗ | ∗ | ||
| 6 week | ||||
| 8 week | ||||
| 12 week |
Table VIII.
Mean and standard deviation of each element intensity
Table IX.
Results of multiple range test at 2 weeks for intensity of Ca (Duncan test)
| Group 1 | Group 2 | Group 3 | |
|---|---|---|---|
| Group 1 (2452.80 ± 160.07) | ∗ | ∗ | |
| Group 2 (2604.00 ± 286.00) | ∗ | ||
| Group 3 (3318.60 ± 202.03) |
Table X.
Results of multiple range test at 4 weeks for intensity of P
| Duncan test) | |||
|---|---|---|---|
| Group 1 | Group 2 | Group 3 | |
| Group 1 (130.00 ± 11.02) | ∗ | ∗ | |
| Group 2 (132.00 ± 6.96) | ∗ | ||
| Group 3 (150.20 ± 10.76) |
Table XI.
Results of multiple range test at 6 weeks for intensity of P (Duncan test)
| Group 1 | Group 2 | Group 3 | |
|---|---|---|---|
| Group 1 (129.80 ± 8.84) | ∗ | ||
| Group 2 (151.80 ± 8.70) | ∗ | ||
| Group 3 (129.60 ± 16.32) |
Table XII.
Results of multiple range test at 8 weeks for intensity of P (Duncan test)
| Group 1 | Group 2 | Group 3 | |
|---|---|---|---|
| Group 1 (130.20 ± 8.32) | ∗ | ∗ | |
| Group 2 (125.40 ± 10.43) | ∗ | ||
| Group 3 (150.60 ± 10.16) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download