Abstract
Statement of problem
The clinical use of electric and electomagnetic fields for fracture healing applications began in the early 1970s. Since then, several technologies have been developed and shown to promote healing of fractures. Developments of these devices have been aided in recent years by basic research and several well controlled clinical trials not only in the medical field but in dentistry.
Purpose
The purpose of this study was to compare alveolar bone reduction following immediate implantation using implants onto which magnets were attached in fresh extracted sockets.
Material and methods
Four mongrel dogs were involved. Full buccal and lingual mucoperiosteal flaps were elevated and third and fourth premolars of the mandible were removed. Implants with magnets and implants without magnets were installed in the fresh extracted sockets and after 3 months of healing the animals were sacrificed. The mandibles were dissected and each implant sites were sampled and processed for histological examination.
Results
The marginal gaps that were present between the implant and walls of the sockets at the implantation stage disappeared in both groups as a result of bone fill and resorption of the bone crest. The buccal bone crests were located apical of its lingual counterparts. At the 12 week interval the mean of marginal bone resorption in the control group was significantly higher than that of the magnet group. The majority of specimens in magnet group presented early bone formation and less resorption of the buccal marginal bone compared to the control group.
Conclusion
Within the limitations of this study, it could be concluded that implants with magnets attached in the early stages of implantation may provide more favorable conditions for early bone formation and reduce resorption and remodeling of marginal bone. (J Korean Acad Prosthodont 2009;47:435-44)
Go to : 
REFERENCES
1.Bassett CA., Pawluk RJ., Pilla AA. Augmentation of bone repair by inductively coupled electromagnetic fields. Science. 1974. 184:575–7.

2.Bassett CAL., Mitchell SN., Norton L., Pilla AA. A nonoperative salvage of surgically resistant pseudoarthoses and nonunions by pulsing electromagnetic fields: A preliminary report. Clin Orthop. 1977. 1245:128–43.
3.Brighton CT., Black J., Friedenberg ZB., Esterhai JL., Day LJ., Connolly JF. A multicenter study of the treatment of non-union with constant direct current. J Bone Joint Surg Am. 1981. 63:2–13.

4.Ryaby JT. Clinical effects of electromagnetic and electric fields on fracture healing. Clin Orthop Relat Res. 1998. 355:S205–15.
5.Yan QC., Tomita N., Ikada Y. Effects of static magnetic field on bone formation of rat femurs. Med Eng Phys. 1998. 20:397–402.

6.Kotani H., Kawaguchi H., Shimoaka T., Iwasaka M., Ueno S., Ozawa H., Nakamura K., Hoshi K. Strong static magnetic field stimulates bone formation to a definite orientation in vitro and in vivo. J Bone Miner Res. 2002. 17:1814–21.
7.Cho YW., Lee SB., Choi BB. The effect of magnetism (neodymium magnet) on activity of osteoblast. J Korean Acad Stomato Func Occl. 2003. 19:185–94.
8.Lee SM., Lee SB., Choi BB. Effect of magnetism (neodymium magnet) on growth factor receptors of osteoblast. J Korean Acad Stomato Func Occl. 2003. 19:87–96.
9.Hwang YT., Lee SB., Choi DG., Choi BB. The change of bone formation according to magnetic intensity of magnet placed into titanium implant specimens. J Korean Acad Prosthodont. 2005. 43:232–45.
10.Park MW., Lee SB., Kwon KR., Choi DG. The effect of magnetism (neodymium magnet) on bone formation around titanium implants inserted into the tibia of rabbit. J Korean Acad Prosthodont. 2005. 43:519–27.
11.Sullivan M., Casey DM., Alberico R., Litwin A., Schaaf NG. Hyperostosis in an orbital defect with craniofacial implants and open-field magnets: a clinical report. J Prosthet Dent. 2007. 97:196–9.

12.Adell R., Lekholm U., Rockler B., Bra � nemark PI. A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int J Oral Surg. 1981. 10:387–416.

13.Atwood DA. Some clinical factors related to rate of resorption of residual ridges. 1962. J Prosthet Dent. 2001. 86:119–25.
14.Atwood DA. Postextraction changes in the adult mandible as illustrated by microradiographs of midsagittal sections and serial cephalometric roentgenograms. J Prosthet Dent. 1963. 13:810–25.

15.Tallgren A. The continuing reduction of the residual alveolar ridges in complete denture wearers: a mixed-longitudinal study covering 25 years. J Prosthet Dent. 1972. 27:120–32.

16.Johnson K. A study of the dimensional changes occurring in the maxilla after tooth extraction-part I. Normal healing. Australian Dent J. 1963. 8:428–33.
17.Johnson K. A study of the dimensional changes occuring in the maxilla following tooth extraction. Australian Dent J. 1969. 14:241–4.
18.Pietrokovski J., Massler M. Alveolar ridge resorption following tooth extraction. J Prosthet Dent. 1967. 17:21–7.

19.Arau 、jo MG., Lindhe J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J Clin Periodontol. 2005. 32:212–8.

20.Arau 、jo MG., Sukekava F., Wennstro ¨m JL., Lindhe J. Ridge alterations following implant placement in fresh extraction sockets: an experimental study in the dog. J Clin Periodontol. 2005. 32:645–52.

21.Botticelli D., Berglundh T., Lindhe J. Hard-tissue alterations following immediate implant placement in extraction sites. J Clin Periodontol. 2004. 31:820–8.

22.Arau 、jo MG., Sukekava F., Wennstro ¨m JL., Lindhe J. Tissue modeling following implant placement in fresh extraction sockets. Clin Oral Implants Res. 2006. 17:615–24.

23.Arau 、jo MG., Wennstro ¨m JL., Lindhe J. Modeling of the buccal and lingual bone walls of fresh extraction sites following implant installation. Clin Oral Implants Res. 2006. 17:606–14.

24.Lazzara RJ. Immediate implant placement into extraction sites: surgical and restorative advantages. Int J Periodontics Restorative Dent. 1989. 9:332–43.
Go to : 
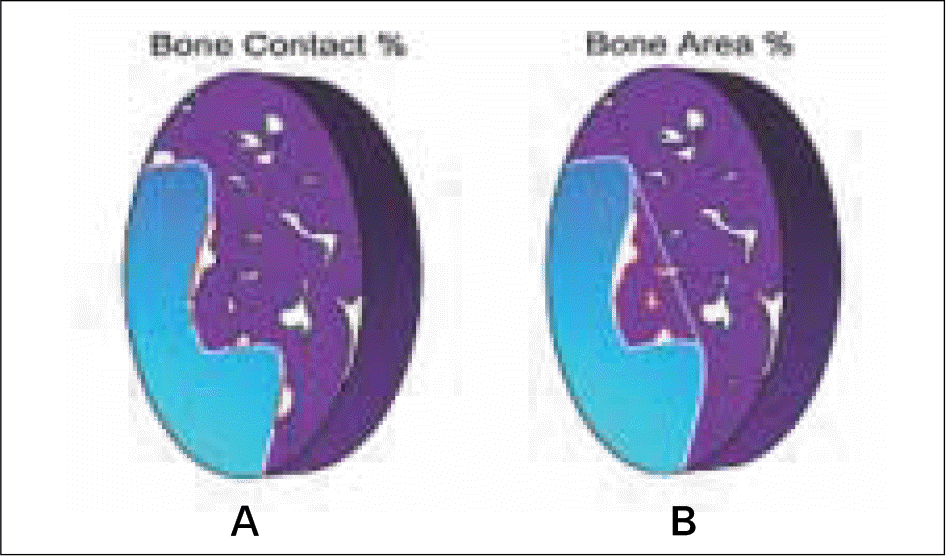 | Fig. 2.Schematic drawing describing bone contact (A) and bone area (B). The percentage of direct bone contact and the amount of bone (bone area), inside the threads were calculated directly in the light microscope. |
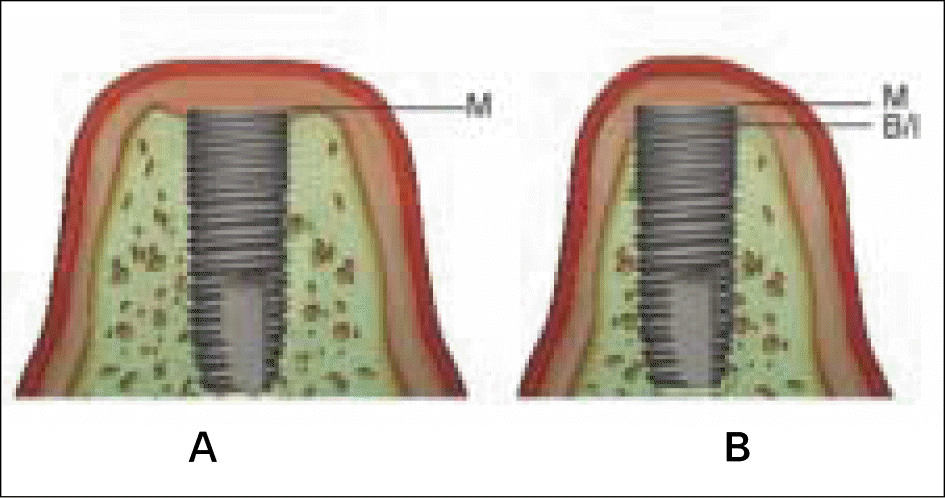 | Fig. 3.Schematic drawing describing the different landmarks between histometric measurements which were performed on implantation (A) and after 12 weeks of healing (B). B/I, marginal level of bone- to-implant contact; M, marginal shoulder of implant. |
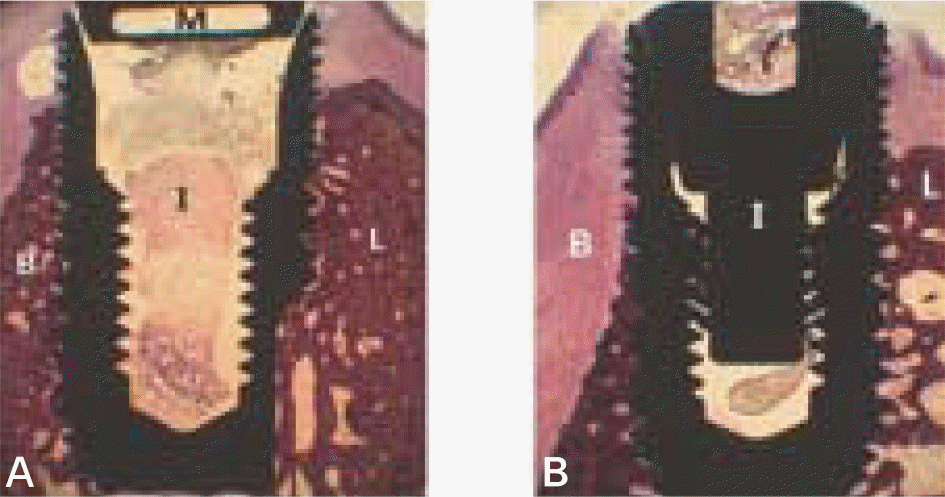 | Fig. 4.Micrographs showing longitudinal section of magnet group (A) and control group (B) after 12 weeks of healing. B, buccal wall; L, lingual wall; I, implant; M, magnet. (H-E staining; original magnification×10). |
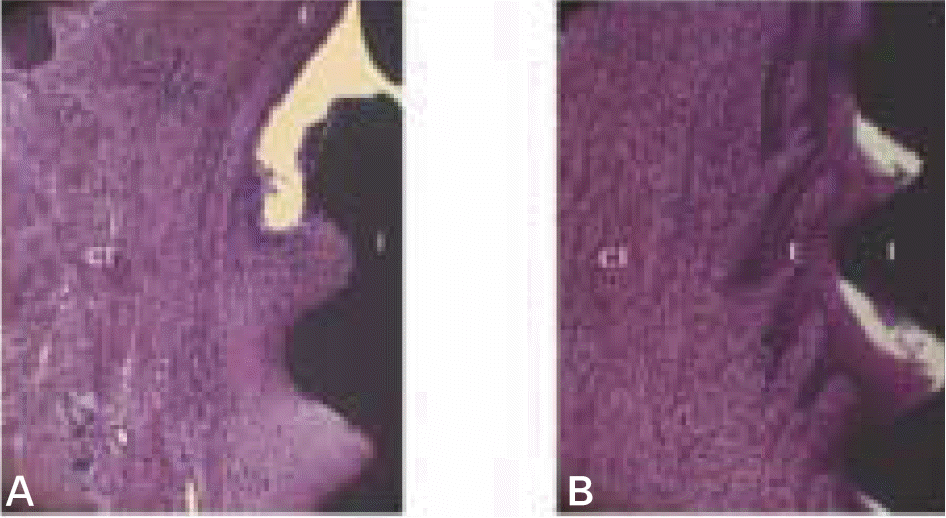 | Fig. 5.Micrographs showing longitudinal buccal section of magnet group (A) and control group (B) after 12 weeks of healing in the cervical region of the implants. CT, connective tissue; E, epithelial tissue; I, implant. (H-E staining; original magnification×40). |
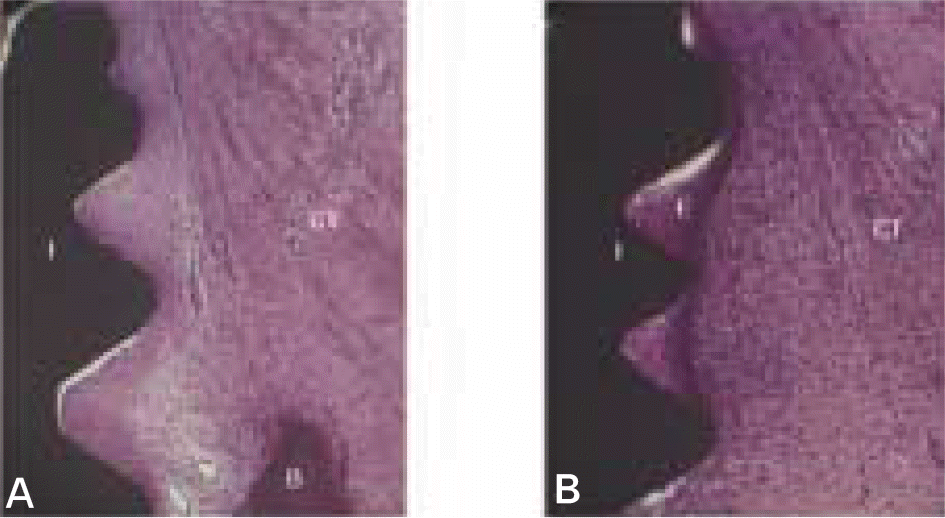 | Fig. 6.Micrographs showing longitudinal lingual section of magnet group (A) and control group (B) after 12 weeks of healing in the cervical region of the implants. B, bone; CT, connective tissue; E, epithelial tissue; I, implant. (H-E staining; original magnification×40). |
 | Fig. 7.Fluorescent images showing longitudinal section of magnet group after 12 weeks of healing. Note that the bone crest is closer to the neck of the implant at the lingual than at the buccal aspect of the implant. B, buccal bone; L, lingual bone; I, implant. (original magnification×100). |
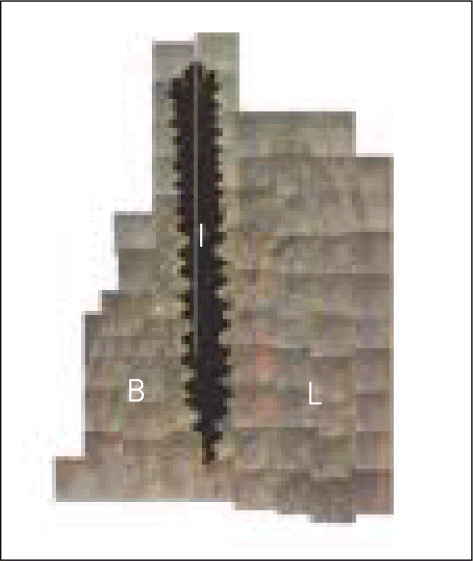 | Fig. 8.Fluorescent images showing longitudinal section of magnet group after 12 weeks of healing. Note that the bone crest is closer to the neck of the implant at the lingual than at the buccal aspect of the implant. B, buccal bone; L, lingual bone; I, implant. (original magnification×100). |
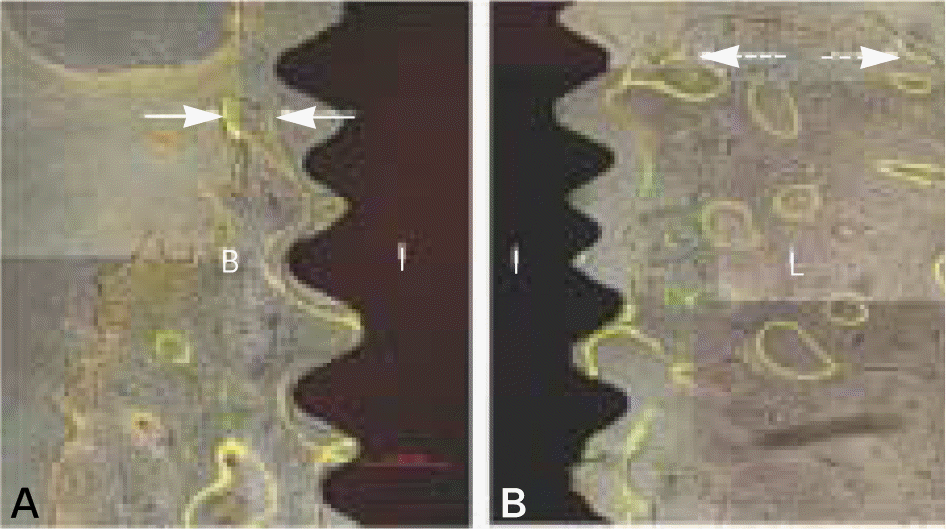 | Fig. 9.Fluorescent images showing longitudinal buccal (A) and lingual (B) section of magnet group after 12 weeks of healing. The solid and dotted arrows indicate the bone resorption after early bone formation at 1 and 6 weeks. B, buccal wall; L, lingual wall; I, implant. (original magnification ×100). |
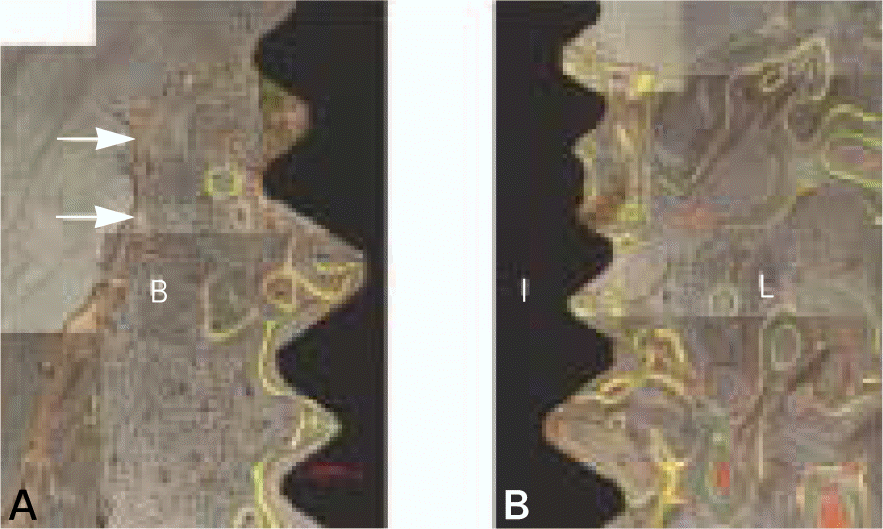 | Fig. 10.Fluorescent images showing longitudinal buccal (A) and lingual (B) sections of control group after 12 weeks of healing. The solid arrows indicate the bone resorption after early bone formation at 1 and 6 weeks. B, buccal wall; L, lingual wall; I, implant. (original magnification×100). |
Table I.
Magnetic intensity at each thread Unit : mT
| Thread | polarity | 1 | 2 | 3 | Mean |
|---|---|---|---|---|---|
| 0 | N | 1.20 | 1.20 | 1.30 | 1.23 |
| 1 | N | 0.10 | 0.10 | 0.20 | 0.13 |
| 2 | S | 1.20 | 1.00 | 1.10 | 1.10 |
| 3 | S | 3.00 | 3.00 | 2.80 | 2.93 |
| 4 | S | 1.00 | 1.10 | 1.00 | 1.03 |
| 5 | S | 0.60 | 0.40 | 0.60 | 0.53 |
| 6 | S | 0.20 | 0.30 | 0.20 | 0.23 |
Table II.
Result distance between ts of histometric m the landmarks (mean easurements (mm) n ± SD)) describing the Unit : mm
| M-B/I | |||
|---|---|---|---|
| Buccal | Lingual | Total | |
| Magnet group | 1.46 ± 0.18∗ | 0.88 ± 0.17∗ | 1.17 ± 0.34∗ |
| Control group | 2.96 ± 0.29∗ | 2.19 ± 0.24∗ | 2.58 ± 0.48∗ |
| Total | 2.21 ± 0.82∗ | 1.54 ± 0.70∗ | |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download