Abstract
Statement of problem
Recently precalcification treatment has been studied to shorten the period of the implant.
Purpose
This study was performed to evaluate the effect of precalcification treatment of TiO2 Nanotube formed on Ti-6Al-4V Alloy.
Material and methods
Specimens of 20 × 10 × 2 mm in dimensions were polished sequentially from #220 to #1000 SiC paper, ultrasonically washed with acetone and distilled water for 5 min, and dried in an oven at 50℃ for 24 hours. The nanotubular layer was processed by electrochemical anodic oxidation in electrolytes containing 0.5 M Na2SO4 and 1.0 wt% NaF. Anodization was carried out using a regulated DC power supply (Kwangduck FA, Korea) at a potential of 20 V and current density of 30 ㎃/㎠ for 2 hours. Specimens were heat-treated at 600℃ for 2 hours to crystallize the amorphous TiO2 nanotubes, and precalcified by soaking in Na2HPO4 solution for 24 hours and then in saturated Ca(OH)2 solution for 5 hours. To evaluate the bioactivity of the precalcified TiO2 nanotube layer, hydroxyapatite formation was investigated in a Hanks’ balanced salts solution with pH 7.4 at 36.5℃ for 2 weeks.
Results
Vertically oriented amorphous TiO2 nanotubes of diameters 48.0 - 65.0 ㎚ were fabricated by anodizing treatment at 20 V for 2 hours in an 0.5 M Na2SO4 and 1.0 NaF solution. TiO2 nanotubes were composed with strong anatase peak with presence of rutile peak after heat treatment at 600℃. The surface reactivity of TiO2 nanotubes in SBF solution was enhanced by precalcification treatment in 0.5 M Na2HPO4 solution for 24 hours and then in saturated Ca(OH)2 solution for 5 hours. The immersion in Hank's solution for 2 weeks showed that the intensity of TiO2 rutile peak increased but the surface reactivity decreased by heat treatment at 600℃.
Go to : 
REFERENCES
1.Crawford GA., Chawla N., Das K., Bose S., Bandyopadhyay A. Microstructure and deformation behavior of biocompatible TiO2 nanotubes on titanium substrate. Acta Biomater. 2007. 3:359–67.

2.Kokubo T., Mijaji F., Kim HM., Nakamura T. Spontaneous apatite formation on chemically surface treated Ti. J Am Ceram Soc. 1996. 79:1127–9.
3.Hanawa T., Ukai H., Murakami K., Asaoka K. Structure of surface-modified layers of calcium-ion-implanted Ti-6Al-4V and Ti-56Ni. Mater Trans JIM. 1995. 36:438–44.
4.Ishizawa H., Fujino M., Ogino M. Mechanical and histological investigation of hydrothermally treated and untreated anodic titanium oxide films containing Ca and P. J Biomed Mater Res. 1995. 29:1459–68.

5.Feng B., Chen JY., Qi SK., He L., Zhao JZ., Zhang XD. Carbonate apatite coating on titanium induced rapidly by precalcification. Biomaterials. 2002. 23:173–9.

6.Balasundaram G., Yao C., Webster TJ. TiO2 nanotubes functionalized with regions of bone morphogenetic protein-2 increases osteoblast adhesion. J Biomed Mater Res A. 2008. 84:447–53.
7.Fini M., Cigada A., Rondelli G., Chiesa R., Giardino R., Giavaresi G., Nicoli Aldini N., Torricelli P., Vicentini B. In vitro and in vivo behaviour of Ca-and P-enriched anodized titanium. Biomaterials. 1999. 20:1587–94.
8.Ishizawa H., Ogino M. Formation and characterization of anodic titanium oxide films containing Ca and P. J Biomed Mater Res. 1995. 29:65–72.

9.Ishizawa H., Ogino M. Characterization of thin hydroxyapatite layers formed on anodic titanium oxide films containing Ca and P by hydrothermal treatment. J Biomed Mater Res. 1995. 29:1071–9.

10.Zhu X., Chen J., Scheideler L., Altebaeumer T., Geis-Gerstorfer J., Kern D. Cellular reactions of osteoblasts to micron- and submicron-scale porous structures of titanium surfaces. Cells Tissues Organs. 2004. 178:13–22.
11.Kasemo B., Lausmaa J. Metal selection and surface characteristics. In: Bra ° nemark PI, Zarb GA, Albrektsson T (eds), Tissue-integrated prostheses, Osseointegration in clinical dentistry. Chicago: Quintessence;1985. p. 99–116.
12.Yang B., Uchida M., Kim HM., Zhang X., Kokubo T. Preparation of bioactive titanium metal via anodic oxidation treatment. Biomaterials. 2004. 25:1003–10.

13.Beranek R., Hidebrand H., Schmuki P. Self-organized porous titanium oxide prepared in H2SO4/HF electrolyte. Electrochemical and Solid-State Letters. 2003. 6:B12–B14.
14.Kaneco S., Chen Y., Westerhoff P., Crittenden JC. Fabrication of uniform size titanium oxide nanotubes: Impact of current density and solution conditions. Scripta Materials. 2007. 56:373–6.

15.Wen HB., Wolke JG., de Wijn Jr., Liu Q., Cui FZ., de Groot K. Fast precipitation of calcium phosphate layers on titanium induced by simple chemical treatments. Biomaterials. 1997. 18:1471–8.

16.Kokubo T., Ito S., Sakka S., Yamamuro T. Formation of a high-strength bioactive glass-ceramic in the system MgO-CaO-SiO2-P2O5. J Mater Soc. 1986. 21:536–40.

17.Kim KN., Bae TS., So JM. Comparison on the calcium phosphate precipitation of NaOH-treated titanium and bioglass-ceramic CaO-P2O5 system. J Korean Res Soc Dent Mater. 2001. 28:247–52.
18.Li P., Ohtsuki C., Kokubo T., Nakanishi K., Soga N., de Groot K. The role of hydrated silica, titania, and alumina in inducing apatite on implants. J Biomed Mater Res. 1994. 28:7–15.

Go to : 
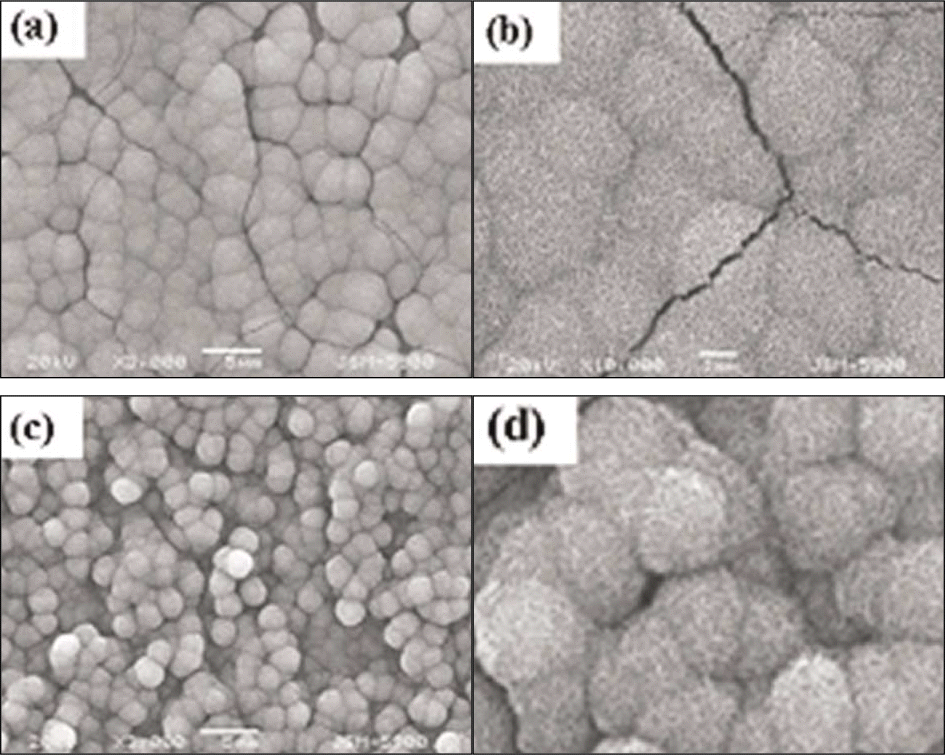
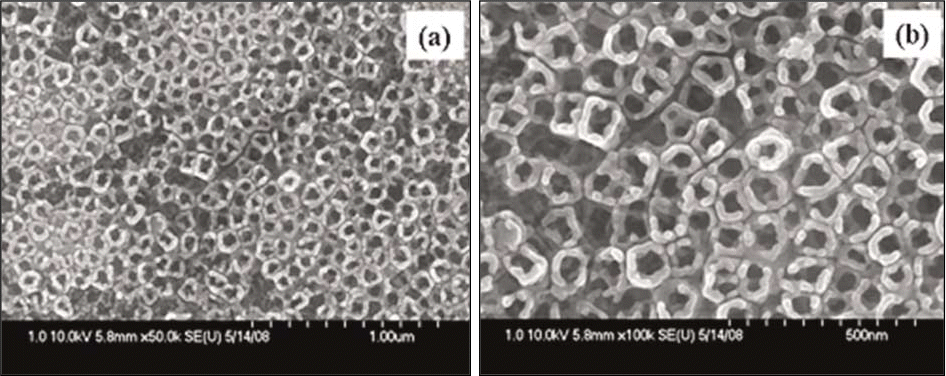 | Fig. 1.FESEM images of TiO2 nanotubes anodized at 20 V for 2 hrs in 0.5 M Na2SO4 and 1 wt% NaF solution. (a) × 50 K; (b) × 100 K. |
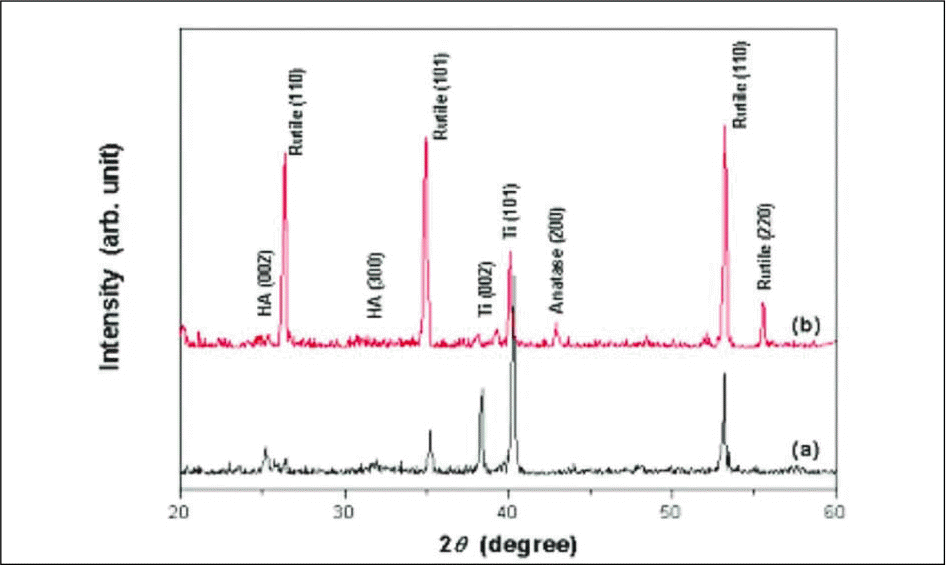 | Fig. 3.XRD patterns of precalcified TiO2 nanotubes after immersed in SBF solution for 2 weeks. (a) Not heat - treated; (b) Heat-treated at 600℃. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download