Abstract
Statements of the problem
Many denture wearers occasionally use denture adhesives to improve denture retention, stability and chewing efficiency. An ideal denture adhesive is nontoxic, non-irritating, and provides comfort to the oral mucosa.
Purpose
The purpose of this study was to evaluate the cytotoxicity and adhesive properties of a selected denture adhesive.
Material and methods
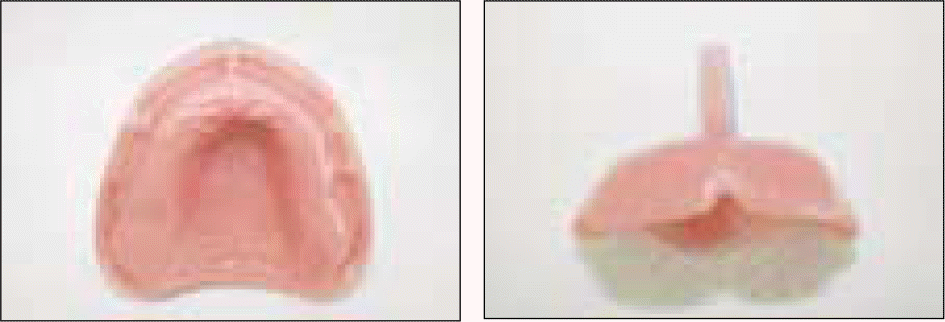
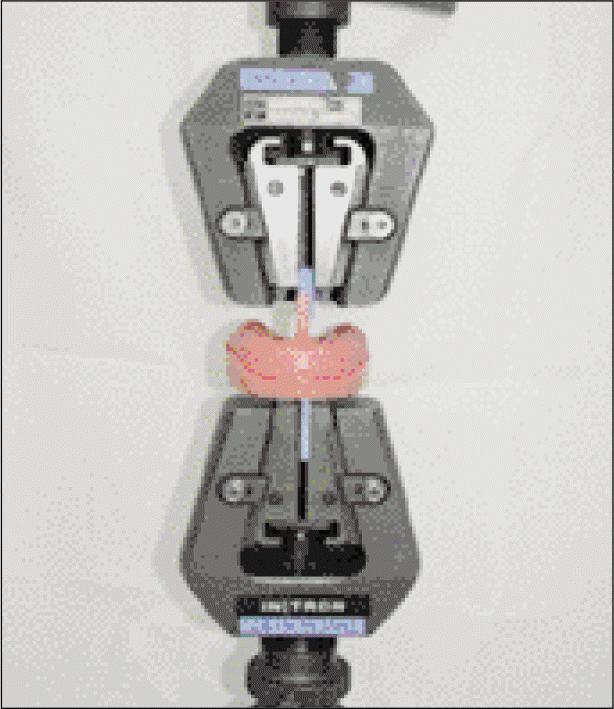
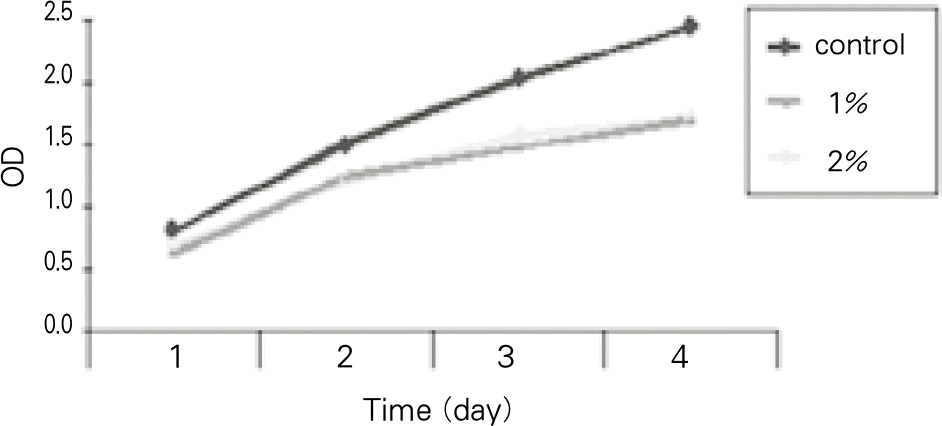
To test cytotoxicity of the selected denture adhesive, mouse fibroblast cells were used in MTT testing. Cytotoxicity was examined according to the concentration of the denture adhesive and incubated for 1 to 4 days. To examine adhesive property, a denture base was fabricated on an edentulous dentiform. The adhesive was applied to the denture base, then tensile bond strength was measured, to evaluate the change in retention during 3 days.
Results andConclusion
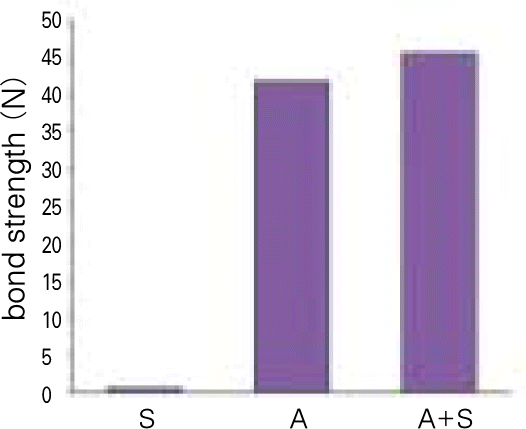
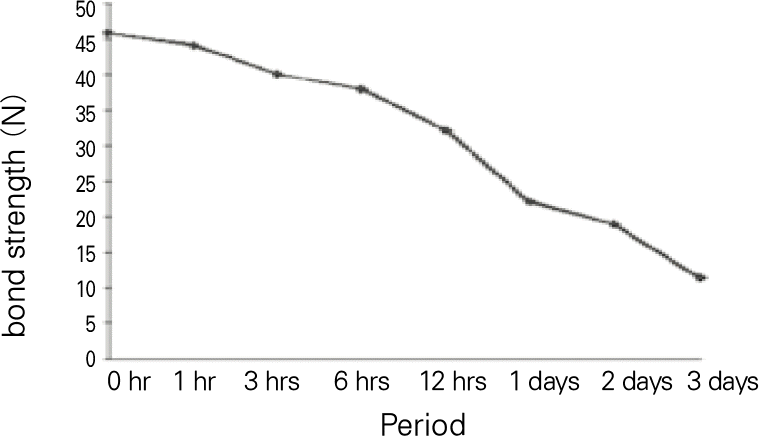
1. 1% and 2% concentration denture adhesive cream had no cytotoxicity. 2. The tensile bond strength of the group with both denture adhesive and artificial saliva was significantly higher than that of the group with only denture adhesive (P < .05). The tensile bond strength of the group with denture adhesive was significantly higher than that of with only artificial saliva (P < .05). 3. The tensile bond strength had no significant change during 1 hour, and then gradually decreased. After 1 day, it decrease to half. Within the limitation of this study, the tested denture adhesive had no cytotoxicilty and was effective in improving denture retention. The adhesive strength began to continuously decrease after 1 hour and it decreased to half at 1 day after application.
Go to : 
REFERENCES
1.Chew CL., Boone ME., Swartz ML., Phillips RW. Denture adhesives: their effects on denture retention and stability. J Dent. 1985. 13:152–9.

4.Norman RD., Stewart GP., Maroso DJ., Gephart JS., Kohut BE. In vitro measurement of vertical denture displacement by denture adhesives. Dent Mater. 1987. 3:342–6.
5.Adisman IK. The use of denture adhesives as an aid to denture treatment. J Prosthet Dent. 1989. 62:711–5.
6.DeVengencie J., Ng MC., Ford P., Iacopino AM. In vitro evaluation of denture adhesives: possible efficacy of complex carbohydrates. Int J Prosthodont. 1997. 10:61–72.
7.Chew CL. Retention of denture adhesives—an in vitro study. J Oral Rehabil. 1990. 17:425–34.
8.Fl � ystrand F., Koppang R., Williams VD., Orstavik J. A method for testing denture adhesives. J Prosthet Dent. 1991. 66:501–4.
9.Al RH., Dahl JE., Morisbak E., Polyzois GL. Irritation and cytotoxic potential of denture adhesives. Gerodontology. 2005. 22:177–83.

10.Gerrard WA., Winter PJ. Evaluation of toothpastes by their ability to assist rehardening of enamel in vitro. Caries Res. 1986. 20:209–16.
11.Koppang R., Berg E., Dahm S., Real C., Fl � ystrand F. A method for testing denture adhesives. J Prosthet Dent. 1995. 73:486–91.

12.Ciapetti G., Cenni E., Pratelli L., Pizzoferrato A. In vitro evaluation of cell/biomaterial interaction by MTT assay. Biomaterials. 1993. 14:359–64.
Go to : 
Table I.
Composition of denture adhesive cream
Table II.
Composition of artificial saliva
| Component | Content |
|---|---|
| distilled water | 2000 ml |
| gastric mucin | 4.4 g |
| NaCl | 0.762 g |
| CaCl2 2H2O | 0.426 g |
| KH2PO4 | 1.476 g |
| KCl | 2.228 g |
| pH | 6.8 |
Table III.
Grades of cytotoxicity corresponding to cell proliferation per centage
| Cell proliferation | Cytotoxicity grade |
|---|---|
| 60 - 100 | Not cytotoxic |
| 30 - 60 | Moderate cytotoxic |
| 0 - 30 | Severe cytotoxic |
Table IV.
MTT cytotoxicity test: the means and standard deviations of optical density
| 1 day | 2 days | 3 days | 4 days | |
|---|---|---|---|---|
| control | 0.82 (0.10) | 1.51 (0.23) | 2.03 (0.16) | 2.44 (0.20) |
| 1% | 0.64 (0.07) | 1.25 (0.25) | 1.48 (0.22) | 1.70 (0.23) |
| 2% | 0.70 (0.10) | 1.21 (0.19) | 1.58 (0.33) | 1.71 (0.30) |
Table V.
Cell proliferation percentage and cytotoxicity grade
| P | 1 day | 2 days | 3 days | 4 days | Cytotoxicity |
|---|---|---|---|---|---|
| Control | 100.00 | 100.00 | 100.00 | 100.00 | Not |
| 1% | 77.84 | 82.93 | 72.82 | 69.80 | Not |
| 2% | 85.06 | 80.33 | 77.68 | 70.11 | Not |
| Cytotoxicity | Not | Not | Not | Not |
Table VI.
The means and standard deviations of tensile bond strength (N) with statistical comparison using one-way ANOVA and LSD post hoc test
| Mean | SD | Statistical comparison | |
|---|---|---|---|
| Saliva | 0.68 | 0.09 | a |
| Adhesive | 41.88 | 3.29 | b |
| Adhesive + Saliva | 45.76 | 3.04 | c |
Table VII.
The means and standard deviations of tensile bond strength (N) according the time with statistical comparison using one-way ANO-VA and LSD post hoc test
| Mean | SD | Statistical comparison | |
|---|---|---|---|
| 0 hr | 45.76 | 3.04 | a |
| 1 hr | 44.12 | 3.38 | a |
| 3 hrs | 40.03 | 3.74 | b |
| 6 hrs | 37.97 | 1.93 | b |
| 12 hrs | 32.08 | 3.03 | c |
| 1 days | 22.04 | 2.87 | d |
| 2 days | 18.33 | 3.84 | d |
| 3 days | 11.33 | 1.15 | e |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download