Abstract
STATEMENT OF PROBLEM
The degree of conversion may influence the ultimate mechanical and physical properties of provisional crown and fixed partial denture materials. The high levels of the unreacted residual monomer may cause deleterious effect on the properties.
PURPOSE
The purpose of this study was to measure the degree of conversion of bis-acrylic based provisional crown and fixed partial denture materials by using an infrared spectroscopic method.
MATERIAL AND METHODS
Chemically activated three bis-acrylic based provisional crown and fixed partial denture materials, LuxaTemp [DMG, Hamburg, Germany], fast set TemPhase [Kerr, Orange, CA, USA] and Protemp 3 Garant [3M-ESPE, St Paul, MN, USA], were investigated by Fourier transform infrared spectrometry (FTIR). The FTIR spectra of the materials tested were immediately obtained after mixing. The specimens were stored under dry conditions and at 23oC for 24 hours, and then the spectra of the materials were also obtained. The degree of conversion (%) was calculated from the spectrum of the absorbance between the aliphatic double bond at 1637 cm-1 and the aromatic double bond at 1608 cm-1 using the baseline method. The data were statistically analyzed using one-way ANOVA and the multiple comparison Scheffe test at the significance level of 0.05.
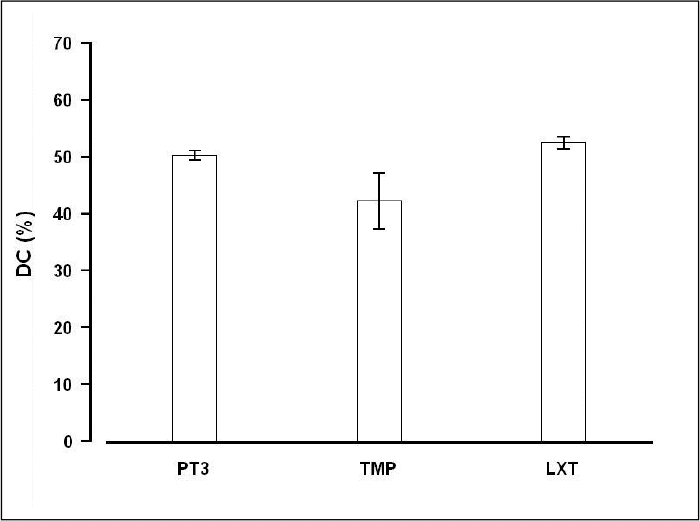
RESULTS
The mean value and standard deviation of the degree of conversion were 52.5 % ± 1.1 %, 50.3 % ± 0.8 %, and 42.3 % ± 4.9 % for LuxaTemp, Protemp 3 Garant and fast set TemPhase, respectively. There was no significant difference between LuxaTemp and Protemp 3 Garant, whereas there was a statistically difference between Protemp 3 Garant and fast set TemPhase, and LuxaTemp and fast set TemPhase (P < .05).
Go to : 
REFERENCES
1.Barron DJ., Rueggeberg FA., Schuster GS. A comparison of monomer conversion and inorganic filler content in visible light-cured denture resins. Dent Mater. 1992. 8:874–7.
2.Asmussen E. Restorative resins. Hardness and strength vs. quantity of remaining double bonds. Scand J Dent Res. 1982. 90:484–9.

3.Asmussen E. Factors affecting the quantity of remaining double bonds in restorative resin polymers. Scand J Dent Res. 1982. 90:490–6.

4.Bradford EW. Case of allergy to methyl-methacrylae. Br Dent J. 1948. 84:195.
6.Giunta JL., Grauer I., Zablotsky N. Allergic contact stomatitis caused by acrylic resin. J Prosthet Dent. 1979. 42:188–90.

7.Ali A., Bates JF., Reynolds AJ., Walker DM. The burning mouth sensation related to the wearing of acrylic dentures: an investigation. Br Dent J. 1986. 161:444–7.

8.Weaver RE., Goebel WM. Reactions to acrylic resin dental prostheses. J Prosthet Dent. 1980. 43:138–42.

9.Ruyter IE., Gyo ¨ ro ¨ si PP. An infrared spectroscopic study of sealants. Scand J Dent Res. 1976. 84:396–400.

10.Smith DC. Acrylic denture base. Residual monomer. Br Dent J. 1958. 105:86–91.
11.Austin AT., Basker RM. The level of residual monomers in acrylic denture base materials. Br Dent J. 1980. 149:281–6.
12.Dogam A., Bek B., Cevik NN., Usanmaz A. The effect of preparation conditions of acrylic denture base materials on the level of residual monomer, mechanical properties and water adsorption. J Dent. 1995. 23:313–8.
13.Koda T., Tsuchiya H., Yamauchi M., Hoshino Y., Takagi N., Kawano J. High-performance liquid chromatographic estimation of elutes from denture base polymers. J Dent. 1989. 17:84–9.
14.Vallittu PK., Miettinium V., Alakuijala P. Residual monomer content and its release into water from denture base materials. Dent Mater. 1995. 11:338–42.

15.Shim JS., Watts DC. Residual monomer concentration in denture-base acrylic resin after an additional, soft-liner, heat-cure cycle. Dent Mater. 1999. 15:296–300.
16.Lamb DJ., Ellis B., Priestley D. The effects of process variables on levels of residual monomer in autopolymerizing dental acrylic resin. J Dent. 1983. 11:80–8.

17.Smith DC., Bains MED. The detection and estimation of residual monomer in polymethyl methacrylate. J Dent Res. 1956. 35:16–24.

18.Antonucci JM., Toth EE. Extent of polymerization of dental resins by differential scanning calorimetry. J Dent Res. 1983. 23:704–7.

19.Miyazaki K., Horibe TJ. Polymerization of multifunctional methacrylates and acrylates. J Biomed Mater Res. 1988. 22:1011–22.

20.Chung KH., Sharma B., Greener EH. Polymerization kinetics in dental acrylics. Dent Mater. 1986. 2:275–8.

21.Eliades GC., Vougiouklakis GJ., Caputo AA. Degree of double bond conversion in light-cured composites. Dent Mater. 1987. 3:19–25.

22.Rueggeberg FA. Determination of resin cure using infrared analysis without an internal standard. Dent Mater. 1994. 10:282–6.

23.Duray SJ., Gilbert JL., Lautenschlager EP. Comparison of chemical analysis of residual monomer in a chemical-cured dental acrylic material to an FTIR method. Dent Mater. 1997. 3:240–5.

24.Ferracane JL., Greener EH. Fourier transform infrared analysis of degree of polymerization in unfilled resins-Methods comparison. J Dent Res. 1984. 63:1093–5.

25.Silikas N., Eliades G., Watts DC. Light intensity effects on resin-composite degree of conversion and shrinkage strain. Dent Mater. 2000. 16:292–6.

26.Stansbury JW., Dickens SH. Determination of double bond conversion in dental resins by near infrared spectroscopy. Dent Mater. 2001. 17:71–9.

27.Venhovan BAM., de Gee AJ., Davidson CL. Polymerization contraction and conversion of light-curing Bis-GMA-based methacrylate resins. Biomaterials. 1993. 14:871–5.

28.Rathbun MA., Craig RG., Hanks CT., Filisko FE. Cytotoxicity of a Bis-GMA dental composite before and after leaching in organic solvents. J Biomed Mater Res. 1991. 25:443–57.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download