Abstract
B cells play a role in graft rejection via several mechanisms. Specifically, B cells produce high-affinity antibodies to alloantigens including allogeneic major histocompatibility complex (MHC) with the help of follicular helper T cells. B cells also function as antigen-presenting cells for alloreactive T cells, resulting in the activation of alloreactive T cells. Conversely, the frequency of regulatory B cells increases under inflammatory conditions and suppresses the rejection process. Here, the differential roles of the major B cell subpopulations (B-1, follicular B, marginal zone B, and regulatory B cells) involved in transplantation rejection are discussed together with their interaction with T cells.
B cells are defined by the expression of B cell receptor (BCR), which is a transmembrane form of immunoglobulin associated with associated signaling chains—Igα and Igβ. To produce extremely various kinds of antibodies (Abs), the germline BCR genes—heavy and light chain genes—undergo dramatic changes such as genetic V(D)J rearrangements, somatic hypermutation, and class switching recombination(1). It is well established that the generation of highly antigen-specific Abs requires the involvement of helper CD4+ T cells and the specific interaction of B and CD4+ T cells leads to the germinal center (GC) reaction and their respective differentiation into GC B cells and follicular helper T cells(23). This T cell-dependent Ab response is performed by follicular (FO) B cells, which are the major population of B cells found in lymphoid tissues and blood. However, besides highly antigen-specific Abs with somatic hypermutations, some clonotypes of Abs against commonly encountered antigens from pathogens and altered self largely retain germline BCR sequences with no or only a few somatic mutations and are produced by innate B cells—B-1 cells and marginal zone (MZ) B cells(45).
B-1 and MZ B cells, two types of innate B cells, have common features such as excellent antigen presentation for T cells and rapid Ab secretion upon infectious events(46). However, they are distinct with respect to the developmental progenitors and anatomical locations. B-1 cells actively migrate from serosal cavities to the spleen and sites of inflammation upon infection(78). On the other hand, mouse MZ B cells stay in the spleen, shuttling between MZ and follicles, in the homeostatic state, and enter into T cell zone and red pulp in response to hematogenous infection(9).
Therefore, three major subsets of B cells form three different layers of Ab responses. B-1 cells produce natural Abs regardless of antigenic challenge, whereas MZ B cells are a kind of preselected B cells for blood-borne pathogens. FO B cells are resting cells that are able to generate highly specific Abs relatively long periods after antigenic challenge.
Development of de novo donor-specific Ab (dnDSA) is an important risk factor for chronic rejection(10). The high affinity dnDSA-expressing B cells are not present before the transplantation and their development requires the affinity maturation from low affinity donor-specific FO B cells. Upon transplantation, the low affinity donor-specific FO B cells would be stimulated by the alloantigens such as donor MHC molecules, but these FO B cells cannot be fully activated and fail to differentiate into GC B cells or plasma cells since helper CD4+ T cells specific for them would be inhibited by the T cell-targeted immunosuppressive drugs such as calcineurin inhibitors(11). Without T cell help under the condition of T cell-targeted immunosuppression, donor antigen-reactive FO B cells undergo cell death or anergy and become unable to present antigenic peptide(1213). However, since new FO B cells including low affinity donor-specific BCRs are continuously produced, the complete tolerance induction of donor-specific B cells can not be established. Upon tapering of immunosuppression, the gradual increase of the dnDSA is observed and when the level of dnDSA increases above a certain threshold level of accommodation, chronic rejection begins(14). The development of high affinity dnDSAs requires the participation of follicular helper T (Tfh) cells. As the differentiation of Tfh cells require the multitude of interactions with B cells through CD40-CD40L, CD28-B7, SLAM-SLAM, ICOS-ICOSL, and more(3), it is interesting to ask how donor-specific Tfh cells are produced in spite of immunosuppression and whether B cell-derived signal can induce signals leading to do the development of donor-specific Tfh cells. The frequency of circulating Tfh cells was shown to be associated with chronic rejection and B cell activation(1516).
Although B cells are helped by CD4+ T cells for high affinity Ab production, B cells are one of important antigen-presenting cells for CD4+ T cells(17). Therefore, the interaction between B and CD4+ T cells are mutually cooperative. T cell stimulatory activity of B cells is usually much poorer than that of dendritic cells (DCs). Upon stimulation with various kinds of pathogens or damage, DCs dramatically change their shape, migratory property, and costimulatory activity, becoming excellent antigen-presenting cells for CD4+ T cells. On the other hand, the expression levels of costimulatory molecules such as MHC class II, CD80, and CD86 is lower in B cells than in DCs. However, antigen presentation of B cells has features distinct from those of DCs(18). Firstly, B cells can proliferate after activation differently from DCs that are destined to die in the lymph node(19). The BCRs they have would be distinct antigen-capturing receptors DCs do not have for continuing antigen presentation. Secondly, B cell interaction with CD4+ T cells can form a positive feedback loop that would sustain prolonged T cell activation including alloreactive T cells in transplantation(20). B cell-specific deletion of MHC class II showed that B cell antigen presentation is critical for T and B cell activation and differentiation(21). Therefore, B cells are weak, but long-lasting antigen-presenting cells for CD4+ T cells when compared to DCs. Since innate B cells are better antigen-presenting cells than FO B cells, two types of innate B cells are thought to have an Ab-independent role in the rejection(46).
Differences between human and mouse immune systems are also appreciated both in B-1 and MZ B cells. In mouse, B-1 cells are clearly recognized by their localization in serosal cavities, expression of CD11b and CD5 (only in B-1a cells), natural Ab secretion, and tonic BCR-mediated signaling due to autoreactivity(522). In human, CD5+ B cells are found in various tissues, and the number of CD5+ B cells is expanded in autoimmune diseases(23). Although these human CD5+ B cells were initially thought to be human B-1 cells(24), the expression of CD5 appeared not be specific for human B-1 cells since some clear B2 and transitional B cells expressed CD5(25). On the other hand, by addressing basic characteristics of mouse B-1 cells in human, CD20+CD27+ CD43+CD70− B cells were shown to be human B-1 cells that were present in both umbilical cord and adult peripheral blood(2627). The antigen presenting function of B-1 cells were noted in a CD11b+ subpopulation of human B-1 cells(28), but their migratory property such as its ability to infiltrate into inflamed tissues such as graft has not been yet reported. Therefore, the full spectrum of human B-1 cells is not addressed yet and the roles of B-1 cells in transplantation needs to be investigated in more detail.
The characteristics of human MZ B cells are also different from those of mouse MZ B cells. The evidence of human MZ B cells came from a large population of CD27+ B cells in peripheral blood(29). These cells were initially thought to be a type of memory IgM+ B cells without class switching recombination since they secreted Ab in the presence of Staphylococcus aureus Cowan strain and IL-2 (3031). However, these subset of B cells were shown to be present even in X-linked hyperIgM patients with no germinal center reaction, suggesting that they are not bona fide memory B cells(32). They were later shown to have many features of mouse MZ B cells although human CD27+ IgM+ B cells have the capacity to recirculate and some somatic hypermutations differently from mouse MZ B cells(33). Human circulating MZ B cells were identified as IgMhigh IgDlowCD23−CD21+CD1c+(34). Mouse and human MZ B cells respond to blood bacteria and also polysaccharide vaccines and this response is not found in newborns since the MZ B cell development takes a few weeks in mouse and about two years in human(35). Besides rapid Ab response to blood-borne pathogens, MZ B cells can carry circulating antigens such as soluble donor-derived antigens to lymphoid tissues where alloreactive T cells and FO B cells are present so that MZ B cells eventually support FO B cell-driven dnDSA production.
Collectively, human B-1 and MZ B cells are unique types of innate B cells that develop and are selected in different ways. Their Ab secretion may contribute in carbohydrate and lipid antigens in transplantation(36), but they have much important Ab-independent functions as antigen-presenting cells for CD4+ T cells.
Regulatory B (Breg) cells are a B cell population that suppress immune responses independent of Ab secretion, but in a manner dependent or not on their IL-10 secretion(373839). They can inhibit expansion of effector T cells and other pro-inflammatory lymphocytes(40) and, therefore, are a potential candidate for cell-based immunotherapy for transplantation(41). Breg cells are identified as CD19+ CD21highCD23highIgMhigh transitional B cells in mouse(37) and CD19+CD24highCD38+ B cells in human(4243) and their development required the presence of SHIP-1(44). In fact, some fraction of B-1 cells also produce IL-10(44), but the immunosuppressive potential of MZ B precursor B-10 cells appears to stronger than that of B-1 cells(38). As the proportion of Breg cells are increased in transplant-tolerant patients(4546), the assessment will be very helpful to monitor the plausibility of graft tolerance. Although B cell depletion therapy is helpful to block alloreactive T cells and dnDSAproducing B cells, caution should be taken to evaluate the danger of Breg cell depletion as well. The BCR specificity of Breg cells is an important question to address, but the difficulty of the identification of Breg cells (IL-10 secretion is the most reliable marker.) makes the study of Breg cells difficult. It is possible that Breg cells are autoreactive similarly to Treg cells(4748).
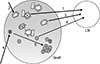
Contrary to previous assumption, B cells are not simply Ab-producing cells, but have several additional important functions such as antigen presentation, pro-inflammatory (such as IL-6) or immunosuppressive (such as L-10) cytokine production, and cooperative interaction with other types of immune cells including T cells. Three major B cell subsets—B-1, FO B, and MZ B cells—form different layers of Ab production. Whereas dnDSA is produced by FO B cells through GC reaction and T-B cell interaction, B-1 and MZ B cells have important roles in triggering alloreactive T cell immune responses (Fig. 1). The plausible scenario is 1) infiltration of B-1 cells into graft, 2) formation of tertiary lymphoid tissues in the graft(49), 3) B-1 cell migration to lymphoid tissues for presentation of antigens to alloreactive T cells, 4) activation of alloreactive T cells in the secondary or tertiary lymphoid tissues, and 5) the interaction between activated alloreactive T cells and FO B cells for the generation of dnDSAs. In this scenario, Breg cells are not depicted, but the contribution of Breg cells is another important factor that determine the fate of rejection or tolerance. It will important to address how we can favor the infiltration of Breg cells, not pro-inflammatory B cells, into the graft.
Figures and Tables
Fig. 1
Sequential events in B cells and dendritic cells (DCs) after transplantation. A plausible scenario of events after transplantation is depicted. 1) migration of resident DCs into draining lymph node, 2) infiltration of newly host-generated DCs, 3) migration of host DCs into draining lymph node, 4) infiltration of B cells into graft (most likely B-1 cells), 5) formation of tertiary lymphoid tissue within the graft (entrance of B2 cells and naïve and memory T cells), and 6) migration of B cells into draining lymph node.
ACKNOWLEDGEMENTS
This work was supported by the research fund from the Basic Science Research Program through the National Research Foundation of Korea (NRF-2015R1D1A1A01056724), which was funded by the Ministry of Science, ICT and Future Planning, Republic of Korea.
References
3. Vinuesa CG, Tangye SG, Moser B, Mackay CR. Follicular B helper T cells in antibody responses and autoimmunity. Nat Rev Immunol. 2005; 5:853–865.

4. Cerutti A, Cols M, Puga I. Marginal zone B cells: virtues of innate-like antibody-producing lymphocytes. Nat Rev Immunol. 2013; 13:118–132.

5. Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol. 2011; 11:34–46.

6. Margry B, Wieland WH, van Kooten PJ, van Eden W, Broere F. Peritoneal cavity B-1a cells promote peripheral CD4+ T-cell activation. Eur J Immunol. 2013; 43:2317–2326.
7. Moon H, Lee JG, Shin SH, Kim TJ. LPS-induced migration of peritoneal B-1 cells is associated with upregulation of CXCR4 and increased migratory sensitivity to CXCL12. J Korean Med Sci. 2012; 27:27–35.

8. Ha SA, Tsuji M, Suzuki K, Meek B, Yasuda N, Kaisho T, et al. Regulation of B1 cell migration by signals through Toll-like receptors. J Exp Med. 2006; 203:2541–2550.

9. Cinamon G, Zachariah MA, Lam OM, Foss FW Jr, Cyster JG. Follicular shuttling of marginal zone B cells facilitates antigen transport. Nat Immunol. 2008; 9:54–62.

10. Wiebe C, Gibson IW, Blydt-Hansen TD, Pochinco D, Birk PE, Ho J, et al. Rates and determinants of progression to graft failure in kidney allograft recipients with de novo donor-specific antibody. Am J Transplant. 2015; 15:2921–2930.

11. Thaunat O, Koenig A, Leibler C, Grimbert P. Effect of immunosuppressive drugs on humoral allosensitization after kidney transplant. J Am Soc Nephrol. 2016; 27:1890–1900.

12. O'Neill SK, Veselits ML, Zhang M, Labno C, Cao Y, Finnegan A, et al. Endocytic sequestration of the B cell antigen receptor and toll-like receptor 9 in anergic cells. Proc Natl Acad Sci U S A. 2009; 106:6262–6267.
13. Fulcher DA, Lyons AB, Korn SL, Cook MC, Koleda C, Parish C, et al. The fate of self-reactive B cells depends primarily on the degree of antigen receptor engagement and availability of T cell help. J Exp Med. 1996; 183:2313–2328.

14. Lynch RJ, Platt JL. Accommodation in organ transplantation. Curr Opin Organ Transplant. 2008; 13:165–170.

15. Shi J, Luo F, Shi Q, Xu X, He X, Xia Y. Increased circulating follicular helper T cells with decreased programmed death-1 in chronic renal allograft rejection. BMC Nephrol. 2015; 16:182.

16. Conlon TM, Saeb-Parsy K, Cole JL, Motallebzadeh R, Qureshi MS, Rehakova S, et al. Germinal center alloantibody responses are mediated exclusively by indirect-pathway CD4 T follicular helper cells. J Immunol. 2012; 188:2643–2652.

17. Vascotto F, Le Roux D, Lankar D, Faure-Andre G, Vargas P, Guermonprez P, et al. Antigen presentation by B lymphocytes: how receptor signaling directs membrane trafficking. Curr Opin Immunol. 2007; 19:93–98.

18. Cascalho MI, Chen BJ, Kain M, Platt JL. The paradoxical functions of B cells and antibodies in transplantation. J Immunol. 2013; 190:875–879.

19. Chen M, Wang J. Programmed cell death of dendritic cells in immune regulation. Immunol Rev. 2010; 236:11–27.

20. Lund FE, Randall TD. Effector and regulatory B cells: modulators of CD4+ T cell immunity. Nat Rev Immunol. 2010; 10:236–247.

21. Giles JR, Kashgarian M, Koni PA, Shlomchik MJ. B cell-specific MHC class II deletion reveals multiple nonredundant roles for B cell antigen presentation in murine lupus. J Immunol. 2015; 195:2571–2579.

22. Hardy RR. B-1 B cells: development, selection, natural autoantibody and leukemia. Curr Opin Immunol. 2006; 18:547–555.

23. Taniguchi O, Miyajima H, Hirano T, Noguchi M, Ueda A, Hashimoto H, et al. The Leu-1 B-cell subpopulation in patients with rheumatoid arthritis. J Clin Immunol. 1987; 7:441–448.

24. Casali P, Burastero SE, Nakamura M, Inghirami G, Notkins AL. Human lymphocytes making rheumatoid factor and antibody to ssDNA belong to Leu-1+ B-cell subset. Science. 1987; 236:77–81.

25. Lee J, Kuchen S, Fischer R, Chang S, Lipsky PE. Identification and characterization of a human CD5+ pre-naive B cell population. J Immunol. 2009; 182:4116–4126.

26. Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70−. J Exp Med. 2011; 208:67–80.

27. Rothstein TL, Griffin DO, Holodick NE, Quach TD, Kaku H. Human B-1 cells take the stage. Ann N Y Acad Sci. 2013; 1285:97–114.

28. Griffin DO, Rothstein TL. A small CD11b(+) human B1 cell subpopulation stimulates T cells and is expanded in lupus. J Exp Med. 2011; 208:2591–2598.

29. Agematsu K, Nagumo H, Yang FC, Nakazawa T, Fukushima K, Ito S, et al. B cell subpopulations separated by CD27 and crucial collaboration of CD27+ B cells and helper T cells in immunoglobulin production. Eur J Immunol. 1997; 27:2073–2079.

30. Clutterbuck EA, Salt P, Oh S, Marchant A, Beverley P, Pollard AJ. The kinetics and phenotype of the human B-cell response following immunization with a heptavalent pneumococcal-CRM conjugate vaccine. Immunology. 2006; 119:328–337.

31. Sanz I, Wei C, Lee FE, Anolik J. Phenotypic and functional heterogeneity of human memory B cells. Semin Immunol. 2008; 20:67–82.

32. Agematsu K, Nagumo H, Shinozaki K, Hokibara S, Yasui K, Terada K, et al. Absence of IgD-CD27(+) memory B cell population in X-linked hyper-IgM syndrome. J Clin Invest. 1998; 102:853–860.

33. Weller S, Mamani-Matsuda M, Picard C, Cordier C, Lecoeuche D, Gauthier F, et al. Somatic diversification in the absence of antigen-driven responses is the hallmark of the IgM+ IgD+ CD27+ B cell repertoire in infants. J Exp Med. 2008; 205:1331–1342.

34. Weill JC, Weller S, Reynaud CA. Human marginal zone B cells. Annu Rev Immunol. 2009; 27:267–285.

35. Pillai S, Cariappa A. The follicular versus marginal zone B lymphocyte cell fate decision. Nat Rev Immunol. 2009; 9:767–777.

36. Irei T, Ohdan H, Zhou W, Ishiyama K, Tanaka Y, Ide K, et al. The persistent elimination of B cells responding to blood group A carbohydrates by synthetic group A carbohydrates and B-1 cell differentiation blockade: novel concept in preventing antibody-mediated rejection in ABO-incompatible transplantation. Blood. 2007; 110:4567–4575.

37. Evans JG, Chavez-Rueda KA, Eddaoudi A, Meyer-Bahlburg A, Rawlings DJ, Ehrenstein MR, et al. Novel suppressive function of transitional 2 B cells in experimental arthritis. J Immunol. 2007; 06. 15. 178:7868–7878.

38. Tedder TF. B10 cells: a functionally defined regulatory B cell subset. J Immunol. 2015; 194:1395–1401.

39. Ray A, Dittel BN. Mechanisms of regulatory B cell function in autoimmune and inflammatory diseases beyond IL-10. J Clin Med. 2017; 6:12.

40. Rosser EC, Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity. 2015; 42:607–612.

41. Goode I, Xu H, Ildstad ST. Regulatory B cells: the new “it” cell. Transplant Proc. 2014; 46:3–8.

42. Flores-Borja F, Bosma A, Ng D, Reddy V, Ehrenstein MR, Isenberg DA, et al. CD19+CD24hiCD38hi B cells maintain regulatory T cells while limiting TH1 and TH17 differentiation. Sci Transl Med. 2013; 5:173ra23.

43. Blair PA, Norena LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, et al. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity. 2010; 32:129–140.

44. Chen Y, Hu F, Dong X, Zhao M, Wang J, Sun X, et al. SHIP-1 deficiency in AID(+) B cells leads to the impaired function of B10 cells with spontaneous autoimmunity. J Immunol. 2017; 199:3063–3073.

45. Li Y, Koshiba T, Yoshizawa A, Yonekawa Y, Masuda K, Ito A, et al. Analyses of peripheral blood mononuclear cells in operational tolerance after pediatric living donor liver transplantation. Am J Transplant. 2004; 4:2118–2125.

46. Newell KA, Asare A, Kirk AD, Gisler TD, Bourcier K, Suthanthiran M, et al. Identification of a B cell signature associated with renal transplant tolerance in humans. J Clin Invest. 2010; 120:1836–1847.

47. Lemoine S, Morva A, Youinou P, Jamin C. Regulatory B cells in autoimmune diseases: how do they work. Ann N Y Acad Sci. 2009; 1173:260–267.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download