Abstract
Renal vein thrombosis is a rare but serious cause of graft loss in kidney transplant recipients that is usually associated with early surgical complications. Here, we report a rare case of sudden development of late onset renal vein thrombosis after kidney transplantation. A 32-year-old man underwent deceased kidney transplantation 2 years prior. Oliguria and pain suddenly developed at the allograft site along with an elevated serum creatinine level. Doppler ultrasound showed absence of venous flow in the transplanted kidney. Magnetic resonance imaging showed thrombosis from the allograft vein to the anastomosis with the left common iliac vein and a swollen allograft kidney. The patient underwent anticoagulation with unfractionated heparin and warfarin. Serum creatinine normalized and renal vein thrombosis disappeared after 3 months of treatment. Late-onset renal vein thrombosis is rare; however, early detection and treatment are very important to restore renal allograft function.
Renal vein thrombosis (RVT) is a rare, but serious cause of graft loss in kidney transplant recipients (KTRs)(1). The incidence of RVT after kidney transplantation (KT) is 0.4%~6%, and most cases occur as an acute event within 2 weeks after KT(2). Most causes of RVT are surgical complications such as compression of the renal vein by hematoma or lymphocele, kinking in the renal vein, and stenosis of the anastomosis site(3). However, late-onset RVT is very rare and can also be associated with underlying disorders such as glomerulonephritis(4), immunosuppressive therapy(5), a hypercoagulable state(6), or extension of iliofemoral deep venous thrombosis(7). Typical clinical presentation of RVT includes sudden onset of oliguria, hematuria, deterioration of allograft function, and a painful, tender, and swollen allograft(8).
We report a case with sudden development of very late-onset RVT after KT.
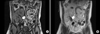
A 32-year-old man was diagnosed with focal segmental glomerulosclerosis due to severe proteinuria. He progressed to end-stage renal disease after 2 years and was started on thrice weekly hemodialysis. He underwent deceased donor KT. We used antithymocyte globulin (ATG) for induction, and tacrolimus, mycophenolate mofetil, and prednisolone for maintenance. Focal segmental glomerulonephritis recurred within a day after KT. He received plasmapheresis nine times in 14 days, and a single dose of rituximab (375 mg/m2). Three weeks after KT, he was discharged with a serum creatinine level of 0.98 mg/dL. However, he required intravenous supplementation of albumin twice a week because of uncontrolled proteinuria. At 19 months after KT, he was readmitted with sudden development of oliguria, elevated serum creatinine, and a painful and swollen allograft. He had a blood pressure of 130/80 mmHg, pulse rate 120 beats per minute, respiration rate 20 breaths per minute, and temperature 37.3℃. He had severe pitting edema in both legs. Laboratory tests showed white blood cell (WBC) count 12,570/mL (81.5% neutrophils), hemoglobin 17.6 g/dL, platelet count 194,000/mL, Na+/K+ 137/4.5 mEq/L, blood urea nitrogen/creatinine 36/2.26 mg/dL (fractional excretion of Na 0.32), C-reactive protein 2.54 mg/dL, serum albumin 2.7 g/dL, and trough level of tacrolimus 7.3 ng/mL. Urinalysis revealed albuminuria (3+), many red blood cells per high-power field (HPF), WBCs 2 to 4/HPF, and a spot urine protein:creatinine ratio of 6.71 g/g. Ultrasonography showed an enlarged allograft kidney with a filling defect in a pulseless main renal vein and increased parenchymal echogenicity (resistance index, 0.75) (Fig. 1). Magnetic resonance imaging (MRI) showed thrombosis from the allograft vein to the anastomosis site of the left common iliac vein and a swollen allograft kidney (Fig. 2). He underwent anticoagulation with unfractionated heparin for 5 days, followed by warfarin. The serum creatinine level decreased to 0.97 mg/dL and the allograft thrombosis disappeared after 3 months of treatment.
Most cases of RVT in KTRs reportedly occur within 2 weeks after KT. Most cases are caused by surgical complications such as extrinsic compression by a perirenal hematoma, lymphocele or other fluid collection, or kinking of vessels(2). The clinical presentation includes sudden onset of oliguria, hematuria, a painful and swollen allograft kidney, and deterioration of renal function(8). Failure to treat RVT of an allograft kidney promptly can lead to irreversible graft dysfunction(9). This was an uncommon case because thrombosis occurred 19 months after KT and the patient experienced recurrent focal segmental glomerulosclerosis. Despite late onset of RVT, he presented with acute oliguria, an elevated serum creatinine level, and a painful and swollen allograft.
The predisposing factors include relapse of nephrotic syndrome(10), technical error(3), high dose steroids(4), delayed graft function recovery(11), thrombocytopenia due to ATG(12), and genetic hypercoagulable states such as antiphospholipid syndrome, autosomal dominant inherited antithrombin deficiency, or mutations of factor V Leiden(613). In the present case, focal segmental glomerulosclerosis recurred after KT and had not been controlled despite aggressive treatment such as plasmapheresis. However, there were no abnormal coagulation factors, antiphospholipid antibodies, or delayed graft function recovery at the time of KT. The renal vein extended from inferior vena cava of deceased donor was connected end-to-side to the external iliac vein without the ligation of internal iliac vein, and the renal artery was connected to the external iliac artery without any technical errors. When ATG was used as an induction agent, thrombocytopenia occurred, but the platelet count recovered a week after stopping ATG.
RVT is diagnosed with Doppler ultrasound; the sonographic image shows absence of venous blood flow with diastolic arterial flow reversal(14). In this case, Doppler ultrasound showed an absence of venous flow in the transplanted kidney. MRI was performed for more precise diagnosis, and showed thrombosis from the allograft kidney vein to the anastomosis site of the left common iliac vein and a swollen allograft kidney.
As prompt intervention is needed to avoid irreversible graft loss, RVT is often treated with medical thrombolytics or mechanical thrombectomy. In this case, we used unfractionated heparin and warfarin, without thrombolytics and mechanical thrombectomy. Allograft function normalized and thrombosis disappeared after 3 months of treatment.
In conclusion, late-onset RVT in KTR is rare and the risk is higher with recurrent glomerulonephritis. Careful monitoring for risk factors of RVT is required; early detection through strong clinical suspicion and prompt treatment are very important to restore renal allograft function.
Figures and Tables
References
1. Irish A. Renal allograft thrombosis: can thrombophilia explain the inexplicable? Nephrol Dial Transplant. 1999; 14:2297–2303.

2. Ripert T, Menard J, Schoepen Y, Nguyen P, Rieu P, Staerman F. Preventing graft thrombosis after renal transplantation: a multicenter survey of clinical practice. Transplant Proc. 2009; 41:4193–4196.

3. Hogan JL, Rosenthal SJ, Yarlagadda SG, Jones JA, Schmitt TM, Kumer SC, et al. Late-onset renal vein thrombosis: a case report and review of the literature. Int J Surg Case Rep. 2015; 6C:73–76.

4. Golden J, Stone RA, Goldberger L. Immune-related renal vein thrombosis in a renal allograft. Ann Intern Med. 1976; 85:612–613.

5. Abramowicz D, Pradier O, Marchant A, Florquin S, De Pauw L, Vereerstraeten P, et al. Induction of thromboses within renal grafts by high-dose prophylactic OKT3. Lancet. 1992; 339:777–778.

6. Irish AB, Green FR, Gray DW, Morris PJ. The factor V Leiden (R506Q) mutation and risk of thrombosis in renal transplant recipients. Transplantation. 1997; 64:604–607.

7. Ramirez PJ, Gohh RY, Kestin A, Monaco AP, Morrissey PE. Renal allograft loss due to proximal extension of ileofemoral deep venous thrombosis. Clin Transplant. 2002; 16:310–313.

8. Asghar M, Ahmed K, Shah SS, Siddique MK, Dasgupta P, Khan MS. Renal vein thrombosis. Eur J Vasc Endovasc Surg. 2007; 34:217–223.

9. Giustacchini P, Pisanti F, Citterio F, De Gaetano AM, Castagneto M, Nanni G. Renal vein thrombosis after renal transplantation: an important cause of graft loss. Transplant Proc. 2002; 34:2126–2127.

10. Li SJ, Tu YM, Zhou CS, Zhang LH, Liu ZH. Risk factors of venous thromboembolism in focal segmental glomerulosclerosis with nephrotic syndrome. Clin Exp Nephrol. 2016; 20:212–217.

11. Bakir N, Sluiter WJ, Ploeg RJ, van Son WJ, Tegzess AM. Primary renal graft thrombosis. Nephrol Dial Transplant. 1996; 11:140–147.

12. Kamel MH, Mohan P, Conlon PJ, Little DM, O'Kelly P, Hickey DP. Rabbit antithymocyte globulin related decrease in platelet count reduced risk of pediatric renal transplant graft thrombosis. Pediatr Transplant. 2006; 10:816–821.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download