INTRODUCTION
Achieving appropriate hepatic inflow is essential for successful liver transplantation. For patients with portal hypertension, a spontaneous splenorenal shunt (SRS) is frequently observed, and should be managed to prevent “portal steal phenomenon.” Inadequate portal flow following living donor liver transplantation (LDLT) is especially an obstacle for liver regeneration, and may lead to graft failure. To address this inadequate flow, various procedures including direct ligation of the SRS, splenectomy, left renal vein ligation (LRVL), and renoportal anastomosis can be applied.
Lee et al.(1) first reported LRVL in a patient with a large SRS during LDLT, and this was widely accepted as a good alternative to prevent portal flow steal. However, the complexity of hemodynamics in the recipient with portal hypertension can cause unexpected results after LRVL. In the present case, a large SRS was detected on pretransplant abdominal computed tomography (CT). Operative procedures including LRVL proceeded well as planned, but posttransplantation abdominal CT revealed remaining portal vein stenosis and the left renal vein and the SRS were filled with a thrombus. Therefore, we reviewed our decision as to whether LRVL was the best choice for our patient and describe better options to prevent unexpected renal and SRS thrombosis after LDLT.
CASE REPORT
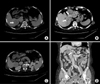
A 54-year-old woman with hepatitis B was referred to our department to manage a 4 cm diameter mass in hepatic segment 6. She was diagnosed with left intrahepatic duct (IHD) stones 25 years ago, and received left hepatic cholangiojejunostomy at another medical center. In 2004, she was admitted to our Emergency Department with symptoms of fever, chills, and abdominal pain. On abdominal CT, multiple IHD stones with IHD dilation and obliterated portal vein, and a SRS were observed. Thereafter, she suffered from iterative biliary sepsis caused by IHD stones, and was managed by conservative therapy, including external drainage using a percutaneous transhepatic biliary drainage or endoscopic stenting. In October 2014, abdominal CT scan, performed during her follow-up, showed a new hepatic mass compatible with hepatocellular carcinoma, multiple IHD stones, an obliterated portal vein with diameter of 6 mm, and a SRS larger than 1 cm (Fig. 1). We scheduled to perform LDLT and planned to ligate the left renal vein to prevent portal flow steal. Pretransplantation laboratory tests showed platelet count of 79×103/µL, total bilirubin of 1.51 mg/dL, prothrombin time of international normalized ratio (INR) 1.48, and serum creatinine of 0.78 mg/dL. The candidate donor was the son of the patient, and the measured graft-to-recipient weight ratio (GRWR) was 1.2.
On laparotomy, severe adhesions as a result of previous surgery and hepatic inflammation made for a complex transplant procedure. The portal vein had poor wall quality with phlebosclerotic changes and was filled with an organized thrombus. We removed the thrombus using an eversion thrombectomy technique, and after ensuring stronger portal flow by clamping the left renal vein, this was followed by an end-to-end anastomosis of the donor portal vein to the confluence of the right and left portal vein of the recipient's portal vein. After reperfusion, we ligated the left renal vein at the junction of the inferior vena cava and the left renal vein with a 2-0 silk tie. Doppler examination showed patent portal flow with velocity of 34 cm/sec. Biliary reconstruction was performed by hepaticojejunostomy using a previous Roux limb.
The patient received antithrombin III and prostaglandin E1 for 7 days after liver transplantation, and antiplatelet prophylaxis with aspirin was started when hemostasis occurred. The immediate postoperative course was uneventful and laboratory tests that were performed 7 days after surgery were satisfactory: aspartate aminotransferase (AST) 24 IU/L, alanine aminotransferase (ALT) 59 IU/L, total bilirubin 0.66 mg/dL, serum creatinine 0.66 mg/dL, and platelet count 80×103/µL. However, dynamic CT showed some unexpected findings (Fig. 2). A left renal vein thrombus developed and extended just proximal to the renal pelvis, preserving the left gonadal vein, and the SRS was occluded by thrombus. Furthermore, portal vein stenosis was observed. Despite these findings, the patient was in a satisfactory condition and abdominal drainage output decreased gradually. Hepatic and renal functions maintained within the normal ranges and the laboratory results performed on postoperative day 20 were as follows: AST 8 IU/L, ALT 28 IU/L, total bilirubin 0.52 mg/dL, INR 0.98, serum creatinine 0.73 mg/dL, and platelet count 216×103/µL. Initially, although we considered placement of a portal stent, on balance, we decided to “wait and see.” The patient is currently doing well without suggestive symptoms 24 months after liver transplantation.
DISCUSSION
LRVL reverses the blood flow from the left renal vein to the portal vein through the SRS and improves portal flow to the allograft(2). Although there are some reports on renal complication after ligation of the left renal vein, it is regarded as a safe procedure without adverse effect on renal function because of alternative routes of renal venous flow such as the lumbar vein, the gonadal vein, and the adrenal vein(3456). In addition, this procedure can be performed with ease because of anatomical simplicity near the confluence of the left renal vein with the inferior vena cava. In the present case, we planned to ligate the left renal vein to manage a large SRS and operative procedures were performed smoothly, gaining grossly adequate portal inflow. However, portal vein stenosis, left renal vein, and SRS thrombus were found on an abdominal CT scan 7 days after liver transplantation. We thought that the left renal vein and SRS thrombus might be caused by stasis of venous flow because of the portal vein stenosis and LRVL. In spite of the left renal vein thrombosis, the serum creatinine level of the recipient was normal, which could be ascribed to preserving the left gonadal vein patency for renal outflow drainage. Moon et al.(7) also showed 4.5% of left renal vein thrombosis rates after LRVL without deterioration of renal function. Initially, we concerned the extension of thrombus to the splenic vein resulting in left-sided portal hypertension or thromboembolic events to the liver, but these events did not occur.
To prevent these unexpected thrombotic events and remaining portal stenosis, we reviewed our decision whether simple ligation of left renal vein for a large SRS was the best choice for the patient and concluded that we had made some mistakes. First, the patient had suffered from hepatitis B and biliary problems for over 25 years, resulting in repeated perihepatic inflammation-induced phlebosclerotic change of the portal vein, and subsequent small portal vein presented for a long time on abdominal CT. Moreover, eversion thrombectomy for an organized thrombus can damage the portal vein wall. Taken together, postoperative remaining portal vein stenosis may be a predictable result. In addition, the patient received sufficient liver graft to tolerate graft-over perfusion arising from additional left renal venous return; volumetric calculations of the donor's right hemiliver, calculated by CT imaging, and actual graft weight donated from her son showed GRWR of 1.2 and 1.12, respectively. Therefore, we had to consider performing a renoportal anastomosis for sufficient portal inflow. Indeed, Golse et al.(8) has suggested a decision algorithm for portal revascularization in case of portal vein thrombosis and SRS, and recommended renoportal anastomosis for patients with complete portal vein thrombosis with SRS, large graft, small portal vein, and minor flow improvement after left renal vein clamping. Second, intraoperative cine-portogram (IOCP) may have been a good option in our case. Moon et al.(9) reported successful management of portal vein stenosis by using IOCP and subsequent portal vein stenting during LDLT, and recommended use of IOCP to overcome the limitation of intraoperative ultrasonography in the evaluation of complicated portal collateral systems and to treat the insufficiency of portal inflow to the liver graft. Although the intraoperative portal inflow was grossly sufficient in our case, IOCP should have been applied to check the effect of LRVL. Furthermore, through this, an additional portal vein stent placement was possible for the treatment of the portal vein stenosis.
After liver transplantation, the recipient might be exposed to the various conditions of hypotension resulting in hypoperfusion of the kidney. In the present case, no event causing renal hypoperfusion occurred during the postoperative course, which can explain the patent left renal outflow through the left gonadal vein despite the renal vein thrombosis. Genzini et al.(10) reported successful LRVL during liver transplantation in a recipient with a single kidney. However, inappropriate ligation of the left renal vein without confirmation of complete control of collateral veins and good portal inflow may be harmful for renal function because of unexpected renal vein thrombosis, especially when these scenarios occur simultaneously with renal hypoperfusion(11).
To sum up, we will perform LRVL again when faced with similar situations because the gross portal inflow was improved after clamping of the left renal vein and eversion thrombectomy. However, IOCP should have been performed to confirm the portal flow and remaining portal stenosis in this case, and we think that subsequent stent placement may prevent thrombosis of the left renal vein and SRS by releasing the portal stenosis.
In conclusion, LRVL is a good simple alternative to control a large SRS during LDLT. However, this procedure should be performed after rigorous evaluation of the anatomic and hemodynamic situation of the recipient.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download