Abstract
A high degree of sensitization to human leukocyte antigen requires more intensive induction therapy; however, this increases vulnerability to opportunistic infections following kidney transplantation. Although recent studies have suggested that combined induction therapy with antithymocyte globulin and rituximab would be more effective in highly sensitized kidney recipients, we experienced a case of near-fatal invasive pulmonary aspergillosis 2 months after combined induction and early rejection therapy for graft dysfunction. Fortunately, the patient recovered with intensive antifungal treatment and lung lobectomy for a necrotic cavity. Antifungal prophylaxis should be considered in cases undergoing intensive induction therapy.
Patients who undergo kidney transplantation (KTP) require life-long immunosuppressive treatment to prevent graft rejection. High-grade sensitization to human leukocyte antigen (HLA) and ABO incompatibility require use of pretransplantation preparative therapy, such as rituximab administration and plasmapheresis. Laftavi et al.(1) reported that combination induction therapy with addition of rituximab to rabbit antithymocyte globulin (rATG) resulted in superior graft survival at 5 years, compared to that using rATG alone in KTP recipients with panel reactive antibody (PRA) levels >50%. Yin et al.(2) described seven patients who received a single dose of 600 mg rituximab (375 mg/m2) infusion on the day before surgery and polyclonal antibody (antithymocyte globulin) on the day of surgery; this protocol reduced the occurrence of postoperative humoral rejection in highly sensitized renal transplant recipients. However, such therapy increases the risk of developing opportunistic infections including invasive pulmonary aspergillosis (IPA). The incidence rate of IPA after KTP is 0.4%, in comparison with 1.3% and 1.9% after heart and liver transplantation, respectively(3). Nevertheless, KTP recipients have the highest burden of posttransplant IPA because KTP is the most frequently performed transplant procedure worldwide. Six- and 12-week survival rates for IPA after KTP were 68.8% and 60.7%, respectively, and 22.1% of survivors experienced graft loss(4). We report a case of near-fatal early IPA 2 months after combined induction therapy using rituximab and antithymocyte globulin, in addition to early rejection therapy for graft dysfunction soon after deceased donor KTP. The patient recovered with intensive antifungal treatment and lung lobectomy for a necrotic cavity, and had good allograft function without intermittent hemodialysis.
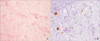
A 57-year-old woman with diabetes mellitus and end-stage renal disease had been on peritoneal dialysis for 4 years, and was changed to intermittent hemodialysis 1 year prior to surgery. She underwent deceased donor KTP with combination induction therapy using rituximab (300 mg/m2) and antithymocyte globulin (1.5 mg/kg/day for 4 days) because of high-grade sensitization to HLA (PRA titer: class I 22%, class II 51%); she was given tacrolimus, mycophenolate mofetil, and prednisolone for immunosuppression. Four days after KTP, she developed an edematous left leg; acute deep vein thrombosis was diagnosed by Doppler ultrasonography; enoxaparin and anticoagulation therapy were started, and an inferior vena cava filter was inserted. Five days after KTP, her urine output decreased to less than 20 mL/hr; her creatinine (Cr) increased from 7.18 to 7.9 and her tacrolimus level was 4.4 ng/mL. She then developed generalized edema. Kidney color Doppler ultrasonography revealed a slightly increased segmental arterial resistive index in the renal allograft (resistive index, 1). For this reason, we could not perform an immediate allograft biopsy. Without biopsy-proven acute antibody-mediated rejection (AMR), even in the absence of donor-specific antibodies (DSA) in a highly sensitized patient with an expected high incidence of early AMR, we started intravenous methylprednisolone (500 mg daily for 3 days), in addition to plasmapheresis and 3 doses of low-dose immunoglobulin (100 mg/kg body weight). Allograft function gradually improved, but allograft biopsy performed 3 weeks after KTP diagnosed calcineurin inhibitor toxicity, rather than rejection. Discharge Cr was 1.4 mg/dL; because of leukopenia (white blood cell [WBC] count 1,500/µL, absolute neutrophil count 1,155), low-dose immunosuppressive agents were given, including tacrolimus (7 mg/day), sirolimus (1 mg/day), and prednisolone (10 mg), but not mycophenolate mofetil. Antibacterial and antiviral prophylaxis included sulfamethoxazole-trimethoprim and valganciclovir after KTP, but antifungal prophylaxis was not given. At 50 days after KTP, she was admitted to the hospital because of a cough with sputum production. On admission, blood pressure was 145/75 mmHg, heart rate was 83 beats per minute, body temperature was 37.6℃, and body mass index was 26.64 kg/m2 (height 160.8 cm, weight 68.2 kg); her lungs were without rales, wheezing, or rhonchi, and there were no other significant findings on physical or neurologic examination. Admission laboratory evaluation showed WBCs 6,950/mm3, C-reactive protein increased to 12.5 mg/dL, Cr 1.7 mg/dL, tacrolimus level 4.5 ng/mL, and hyperglycemia (random glucose 432 mg/dL). Chest X-ray showed consolidation and a lesion in the right lower lobe. Further investigation was needed to confirm the diagnosis. High-resolution computed tomography (CT) showed fibrotic consolidation and a peripheral ground glass opacity pattern in the right lower lobe (Fig. 1A). We started antibiotic therapy (piperacillin, tazobactam) pending the differential workup for pneumonia in early post-KTP patients, to rule out Pneumocystis carinii (PCP), cytomegalovirus (CMV), IPA, bacterial pneumonia, and pulmonary tuberculosis (TB), despite the low probability. The sputum culture and stain for acid-fast bacilli and the blood culture were negative; the CMV polymerase chain reaction (PCR), PCP PCR, and TB PCR were negative, but a positive test for Aspergillus antigen (1.39) was reported on day 7. Although bronchoscopy did not identify a lesion, bronchoalveolar lavage (BAL) and sputum cytology also found Aspergillus. We started voriconazole as an antifungal agent and reduced tacrolimus from 7 mg/day (3.5 mg twice daily) to 2 mg/day. The tacrolimus level was 15.8 ng/mL, so we gradually reduced all immunosuppressant agents. However, her chest X-ray showed a worsening pleural effusion. The effusion was treated by percutaneous drain insertion and the exudate was analyzed. We then added micafungin. However, she needed intubation and mechanical ventilation in the intensive care unit for 5 days, and all immunosuppressive agents were stopped. The Cr decreased from 1.7 to 1.08. As soon as her condition improved, we restarted tacrolimus 0.5 mg and added mycophenolate mofetil and prednisolone 5 mg on day 20. On day 29, the pleural effusion was found to be increasing rapidly, and we performed a percutaneous needle biopsy that was reported as necrotizing granulomatous inflammation with Aspergillus (Fig. 2). Therefore, we diagnosed primary antifungal therapy failure and changed to secondary agents, including combined amphotericin B and anidulafungin; we again reduced and gradually stopped the immunosuppressive agents, and only used maintenance prednisolone 7.5 mg. Thereafter, Cr increased from 1.08 to 3.6 mg/dL, and graft function worsened; we restarted intermittent hemodialysis for fluid volume control. On day 82, follow-up chest CT showed an increasing right loculated pleural effusion (Fig. 1B) and a necrotic cavitary lesion, with the potential for development of a bronchopleural fistula (Fig. 1C). On day 101, right lower lung lobectomy was performed, and an Aspergillus lesion was removed; we stopped antifungal agents 40 days after surgery. Graft function improved and Cr decreased to 1.4 mg/dL, with no further need for hemodialysis (Fig. 3). A low tacrolimus level (1.5 to 2.9 ng/mL) was maintained.
Aspergillus is introduced to the lower respiratory tract by inhalation of infectious spores in patients with severe illness. The diagnosis of IPA remains challenging, and early diagnosis of IPA in severely immunocompromised patients is difficult; a high index of suspicion is necessary in patients with risk factors. Symptoms are nonspecific and patients may present with fever, cough, sputum, and pleuritic chest pain, as well as hemoptysis in neutropenic patients. The gold standard in the diagnosis of IPA is histopathological examination of lung tissue obtained by thoracoscopic or open lung biopsy(5). Bronchoscopy with BAL is helpful, and recent advances in the diagnosis of IPA include detection of Aspergillus antigens in body fluids; these include galactomannan and β-D glucan.
The major risk factor for IPA is immunodeficiency, which occurs with neutropenia, hematopoietic stem cell transplantation, solid organ transplantation, prolonged therapy with high dose corticosteroids, hematological malignancy, cytotoxic therapy, advanced acquired immune deficiency syndrome, and chronic granulomatous disease(678). The present case was a kidney transplant recipient, with neutropenia and steroid therapy as added risk factors, as well as hyperglycemia; all could increase vulnerability to infection. Almost half of the episodes of IPA occur within the first 3 to 6 months after transplantation, and this patient was diagnosed in the first 2 months after transplantation. Risk factors for early IPA within the first 180 days after KTP include pretransplant chronic obstructive pulmonary disease, impaired graft function, bloodstream infection, and acute graft rejection within 3 months prior to the diagnosis of IPA(9). Combined induction therapy using rituximab and antithymocyte globulin, with early rejection therapy for graft dysfunction, including plasmapheresis and low-dose immunoglobulin, contributed to near-fatal IPA in this case. Kamar et al.(10) found that with rituximab therapy, 9.1% of KTP patients died due to opportunistic infectious disease, and the rate was significantly higher than in a control group (1.6%). Chung et al.(11) investigated the effect of combined use of rituximab and pretransplantation plasmapheresis on posttransplant infection. The overall prevalence of infection was significantly higher and the infection-free survival rate was lower in the combined rituximab and plasmapheresis group than in those receiving only rituximab or in controls. Fungal infections developed only in the rituximab plus plasmapheresis group; after antirejection therapy, a significantly higher infection rate developed in the rituximab plus plasmapheresis group than in controls (P<0.05)(11). Combination therapy resulted in severe and sustained impairment of humoral immunity in this case, and can result in a higher overall incidence of infection including IPA. Early AMR can be severe and a major cause of early graft loss. Thus, we recommend aggressive, early treatment of AMR in most cases. We also need to obtain a biopsy and serum DSA for differential diagnosis, to determine increasing Cr is due to acute rejection, dehydration, or an elevated tacrolimus level. If AMR is strongly suspected, we should treat preemptively, and can stop therapy when AMR is ruled out. However, in this case, we could not obtain an immediate allograft biopsy to document AMR, because of anticoagulation therapy with bleeding risk in the first few days after transplantation. Because this was a highly sensitized patient with an expected high incidence of early AMR, we started plasmapheresis, low-dose intravenous immunoglobulin (IVIG), and steroids, which increase vulnerability to opportunistic infections. However, another treatment option that combines a steroid pulse with highdose IVIG is less likely to lead to opportunistic infection. Common adverse events associated with IVIG include mild fever, chills, and headache; rare, serious side effects such as hemolytic anemia, aseptic meningitis, anaphylactic reactions, and vascular thrombosis have been reported(12). As in this case, if allograft biopsy cannot be obtain for suspected AMR, high-dose IVIG may be a better option than combined plasmapheresis and steroid pulse therapy, to reduce opportunistic infection risk. Lopez-Medrano et al.(4) assessed risk factors for graft loss among survivors at 12 weeks after the diagnosis of IPA. The use of acute renal replacement therapy and need for intensive care unit admission, either within the 3 months prior to or at the time of diagnosis of IPA, in addition to use of amphotericin B-containing regimens, were risk factors(4). Graft function in this patient worsened and intermittent hemodialysis was required during amphotericin therapy and prior to control of IPA; however, the patient gradually recovered and she regained good allograft function without need for intermittent hemodialysis.
Despite the use of several new antifungal agents, treatment of IPA remains difficult and mortality rates are still high. Thus, therapy should start as soon as there is clinical suspicion of IPA. A new broad-spectrum triazole, voriconazole, has been approved for initial treatment of IPA, and is currently considered the treatment of choice; another broad spectrum triazole, posaconazole, is effective and safe as salvage therapy in patients with IPA refractory to standard treatment(13). The combination of caspofungin and liposomal amphotericin B as salvage therapy showed an overall response rate of 42%(14). The duration of IPA therapy should be individualized for the patient's clinical and radiological response. The treatment is often prolonged, lasting several months to >1 year. Surgical resection generally plays a limited role in management of IPA, but is used with invasion of bone, epidural abscesses, massive hemoptysis, or involvement of the great vessels or pericardium(15).
There have been other case reports of kidney transplant recipients with invasive Aspergillus infections of the lung, liver, and skin without allograft failure(161718), that required removal of the infected allograft. However, in this case, near-fatal invasive pulmonary Aspergillus infection and allograft failure requiring hemodialysis were concurrently treated with antifungal agents and surgery, as well as by careful stopping and restarting immunosuppressant agents. This suggests that antifungal prophylaxis, including intensive combination induction therapy, should be considered for those at higher risk of invasive aspergillosis.
Current data support the use of antifungal prophylaxis in liver, lung, small bowel, heart, and pancreatic solid organ transplant recipients. Because of the rarity of cases and lack of data, antifungal prophylaxis after KTP is not currently recommended(19), but there are patients who seem to be at increased risk. Therefore, a strategy is needed to identify those patients at high risk for IPA. Panackal et al.(20) reported that the factors associated with increased risk for aspergillosis included graft failure requiring prolonged hemodialysis and extended posttransplant corticosteroid use. Linden et al.(21) reported that initial antilymphocyte induction therapy and/or treatment of rejection increased risk for invasive aspergillosis. Therefore, as in our case, highly-sensitized kidney transplant recipients who receive intensive combination induction therapy should be considered for antifungal prophylaxis, especially in the presence of graft failure requiring prolonged hemodialysis, or extended posttransplant corticosteroid use.
Figures and Tables
 | Fig. 1(A) On hospital day (HD) 1, high-resolution computed tomography (CT) shows fibrotic consolidation and a peripheral ground glass opacity in the right lower lobe. (B, C) Follow-up enhanced chest CT on HD 82. (B) It shows increasing right loculated pleural effusion. (C) It shows a necrotic cavitary lesion at risk of developing a bronchopleural fistula. |
References
1. Laftavi MR, Pankewycz O, Feng L, Said M, Patel S. Combined induction therapy with rabbit antithymocyte globulin and rituximab in highly sensitized renal recipients. Immunol Invest. 2015; 44:373–384.

2. Yin H, Wan H, Hu XP, Li XB, Wang W, Liu H, et al. Rituximab induction therapy in highly sensitized kidney transplant recipients. Chin Med J (Engl). 2011; 124:1928–1932.
3. Cornet M, Fleury L, Maslo C, Bernard JF, Brucker G. Invasive Aspergillosis Surveillance Network of the Assistance Publique-Hôpitaux de Paris.Epidemiology of invasive aspergillosis in France: a six-year multicentric survey in the Greater Paris area. J Hosp Infect. 2002; 51:288–296.

4. Lopez-Medrano F, Fernandez-Ruiz M, Silva JT, Carver PL, van Delden C, Merino E, et al. Clinical presentation and determinants of mortality of invasive pulmonary aspergillosis in kidney transplant recipients: a multinational cohort study. Am J Transplant. 2016; 16:3220–3234.

5. Ruhnke M, Bohme A, Buchheidt D, Donhuijsen K, Einsele H, Enzensberger R, et al. Diagnosis of invasive fungal infections in hematology and oncology: guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO). Ann Hematol. 2003; 82:Suppl 2. S141–S148.
6. Soubani AO, Chandrasekar PH. The clinical spectrum of pulmonary aspergillosis. Chest. 2002; 121:1988–1999.

7. Gerson SL, Talbot GH, Hurwitz S, Strom BL, Lusk EJ, Cassileth PA. Prolonged granulocytopenia: the major risk factor for invasive pulmonary aspergillosis in patients with acute leukemia. Ann Intern Med. 1984; 100:345–351.

8. Segal BH, Walsh TJ. Current approaches to diagnosis and treatment of invasive aspergillosis. Am J Respir Crit Care Med. 2006; 173:707–717.

9. Lopez-Medrano F, Silva JT, Fernandez-Ruiz M, Carver PL, van Delden C, Merino E, et al. Risk factors associated with early invasive pulmonary Aspergillosis in kidney transplant recipients: results from a multinational matched case-control study. Am J Transplant. 2016; 16:2148–2157.

10. Kamar N, Milioto O, Puissant-Lubrano B, Esposito L, Pierre MC, Mohamed AO, et al. Incidence and predictive factors for infectious disease after rituximab therapy in kidney-transplant patients. Am J Transplant. 2010; 10:89–98.

11. Chung BH, Yun JT, Ha SE, Kim JI, Moon IS, Choi BS, et al. Combined use of rituximab and plasmapheresis pretransplant increases post-transplant infections in renal transplant recipients with basiliximab induction therapy. Transpl Infect Dis. 2013; 15:559–568.

13. Kousha M, Tadi R, Soubani AO. Pulmonary aspergillosis: a clinical review. Eur Respir Rev. 2011; 20:156–174.

14. Kontoyiannis DP, Hachem R, Lewis RE, Rivero GA, Torres HA, Thornby J, et al. Efficacy and toxicity of caspofungin in combination with liposomal amphotericin B as primary or salvage treatment of invasive aspergillosis in patients with hematologic malignancies. Cancer. 2003; 98:292–299.

15. Moreau P, Zahar JR, Milpied N, Baron O, Mahe B, Wu D, et al. Localized invasive pulmonary aspergillosis in patients with neutropenia. Effectiveness of surgical resection. Cancer. 1993; 72:3223–3226.

16. Park J, Hwang E, Park S, Kim H, Kim H. Simultaneous lung and liver Aspergillus in a kidney transplant recipient. J Korean Soc Transplant. 2012; 26:202–206.

17. Park SJ. A case of successful treatment of cutaneous Aspergillosis with voriconazole at the low cyclosporine trough level in a renal transplant. J Korean Soc Transplant. 2010; 24:35–39.

18. Na HH, Hong SW, Kim MC, Kang YK, Yoon YC, Koh HI. A case of invasive Aspergillosis in transplanted kidney and perirenal area. J Korean Soc Transplant. 2008; 22:135–137.
19. Brizendine KD, Vishin S, Baddley JW. Antifungal prophylaxis in solid organ transplant recipients. Expert Rev Anti Infect Ther. 2011; 9:571–581.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download