Abstract
Background:
The shortage of human hearts for allotransplantation makes xenotransplantation a possible option for controllable organ providers. To detect acute xenograft rejection, invasive biopsy seems inevitable; however, this occasionally results in poor incision wound healing or infection. To date, no method of noninvasive imaging for early detection of xenograft rejection has been established. We hypothesized that ultrasound speckle tracking would better detect xenograft failure than routine left ventricular ejection fractions (EF).
Methods:
From August 2013 to July 2015, a total of six cardiac heterotopic xenotransplants ( 1, 3-galactosyltransferase gene-knockout porcine heart) into cynomolgus monkeys were monitored with echocardiography every 3 to 7 days. M-mode and two-dimensional (2D)-EF measurements and myocardial strain analyses were performed. Cardiac xenograft pathology was reviewed from the immediate postoperative biopsy, as well as the necropsy.
Results:
Myocardial speckle tracking analysis was feasible in all six cases. The longest survival was 43 days. Only one pathology-proven immunologic rejection occurred. Cardiac xenograft failure appeared as two types: a dilated pattern with decreased EF or a myocardial-thickening pattern with preserved EF. Both antibody-mediated rejection (n=1) and sepsis-induced myocardial dysfunction (n=2) revealed decreased radial or circumferential strains, but normal-range EF. Xenograft functional decline was significant with respect to radial or circumferential strain (P=0.028), but not to conventional M-mode or 2D-EFs (P=0.600, P=0.340, respectively).
Go to : 
REFERENCES
1). Kuwaki K., Tseng YL., Dor FJ., Shimizu A., Houser SL., Sanderson TM, et al. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2005. 11:29–31.
2). Mohiuddin MM., Corcoran PC., Singh AK., Azimzadeh A., Hoyt RF Jr., Thomas ML, et al. B-cell depletion extends the survival of GTKO.hCD46Tg pig heart xenografts in baboons for up to 8 months. Am J Transplant. 2012. 12:763–71.
3). Mohiuddin MM., Singh AK., Corcoran PC., Hoyt RF., Thomas ML 3rd., Ayares D, et al. Genetically engineered pigs and target-specific immunomodulation provide significant graft survival and hope for clinical cardiac xenotransplantation. J Thorac Cardiovasc Surg. 2014. 148:1106–13.

4). Ferferieva V., Van den Bergh A., Claus P., Jasaityte R., La Gerche A., Rademakers F, et al. Assessment of strain and strain rate by two-dimensional speckle tracking in mice: comparison with tissue Doppler echocardiography and conductance catheter measurements. Eur Heart J Cardiovasc Imaging. 2013. 14:765–73.

5). Shi J., Pan C., Shu X., Sun M., Yang Z., Zhu S, et al. The role of speckle tracking imaging in the noninvasive detection of acute rejection after heterotopic cardiac transplantation in rats. Acta Cardiol. 2011. 66:779–85.

6). Pieper GM., Shah A., Harmann L., Cooley BC., Ionova IA., Migrino RQ. Speckle-tracking 2-dimensional strain echocardiography: a new noninvasive imaging tool to evaluate acute rejection in cardiac transplantation. J Heart Lung Transplant. 2010. 29:1039–46.

7). Mingo-Santos S., Monivas-Palomero V., Garcia-Lunar I., Mitroi CD., Goirigolzarri-Artaza J., Rivero B, et al. Usefulness of two-dimensional strain parameters to diagnose acute rejection after heart transplantation. J Am Soc Echocardiogr. 2015. 28:1149–56.
8). Ambardekar AV., Alluri N., Patel AC., Lindenfeld J., Dorosz JL. Myocardial strain and strain rate from speckle-tracking echocardiography are unable to differentiate asymptomatic biopsy-proven cellular rejection in the first year after cardiac transplantation. J Am Soc Echocardiogr. 2015. 28:478–85.

9). Kim H., Chee HK., Yang J., Hwang S., Han KH., Kang J, et al. Outcomes of alpha 1,3-GT-knockout porcine heart transplants into a preclinical nonhuman primate model. Transplant Proc. 2013. 45:3085–91.

10). Mohiuddin MM., Reichart B., Byrne GW., McGregor CG. Current status of pig heart xenotransplantation. Int J Surg. 2015. 23(Pt B):234–9.

11). Stewart S., Winters GL., Fishbein MC., Tazelaar HD., Kobashi-gawa J., Abrams J, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005. 24:1710–20.

12). Behr TM., Feucht HE., Richter K., Reiter C., Spes CH., Pongratz D, et al. Detection of humoral rejection in human cardiac allografts by assessing the capillary deposition of complement fragment C4d in endomyocardial biopsies. J Heart Lung Transplant. 1999. 18:904–12.

13). Lang RM., Badano LP., Mor-Avi V., Afilalo J., Armstrong A., Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015. 28:1–39. .e14.

14). Devereux RB., Alonso DR., Lutas EM., Gottlieb GJ., Campo E., Sachs I, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986. 57:450–8.

15). Cameli M., Mondillo S., Solari M., Righini FM., Andrei V., Contaldi C, et al. Echocardiographic assessment of left ventricular systolic function: from ejection fraction to torsion. Heart Fail Rev. 2016. 21:77–94.

16). Abbott CP., Dewitt CW., Creech O Jr. The transplanted rat heart: histologic and electrocardiographic changes. Transplantation. 1965. 3:432–45.
17). Chen RH., Kadner A., Adams DH. Monitoring pig-to-primate cardiac xenografts with live Internet images of recipients and xenograft telemetric signals: histologic and immunohistochemical correlations. J Heart Lung Transplant. 2000. 19:591–7.

18). Horvath KA., Corcoran PC., Singh AK., Hoyt RF., Carrier C., Thomas ML 3rd, et al. Left ventricular pressure measurement by telemetry is an effective means to evaluate transplanted heart function in experimental heterotopic cardiac xenotransplantation. Transplant Proc. 2010. 42:2152–5.

19). Zhou M., Hara H., Dai Y., Mou L., Cooper DK., Wu C, et al. Circulating organ-specific microRNAs serve as biomarkers in organ-specific diseases: implications for organ allo- and xeno-transplantation. Int J Mol Sci. 2016. 17:E1232.

20). Badano LP., Miglioranza MH., Edvardsen T., Colafranceschi AS., Muraru D., Bacal F, et al. European Association of Cardiovascular Imaging/Cardiovascular Imaging Department of the Brazilian Society of Cardiology recommendations for the use of cardiac imaging to assess and follow patients after heart transplantation. Eur Heart J Cardiovasc Imaging. 2015. 16:919–48.

Go to : 
 | Fig. 1.A donor heart (41.6 g) from a transgenic pig ( 1,3-galactosyltransferase gene-knockout, blood type A, 4-week-old, 6.7 kg) was transplanted into a cynomolgus monkey (blood type A, 5.5-year-old, 6.7 kg). Case number 3, pictured here, survived 43 days. (A, B) Hematoxylin and eosin staining of donor left ventricular walls at necropsy shows severe congestion, hemorrhages, myocyte necrosis, conspicuous endothelial cell changes, intravascular mononuclear cells (×400). No cellular rejection is present (International Society of Heart and Lung Transplantation [ISHLT] acute cellular rejection grade 0R). (C) Immunoglobulin G (IgG) staining of the donor left ventricular myocardium shows a positive stain in the necrotic area (×400). (D) Diffuse, multifocal capillary C4d staining with strong intensity of the corresponding specimen confirmed antibody-mediated rejection (ISHLT antibody mediated rejection) (×400). |
 | Fig. 2.A donor heart (37.5 g) from a transgenic pig ( 1,3-galactosyltransferase gene-knockout, blood type A, 6.4 kg) expressing human complement regulatory protein CD46 was transplanted into a cynomolgus monkey (blood type A, 5.3 kg). Case number 4, pictured here, survived 38 days. An M-mode tracing echocardiogram of the mid-level of the left ventricular (LV) short axis view compared with (A) immediate postoperative and (B) just before expiry. Two-dimensional echocardiograms of the mid-level LV short axis view at the end-systolic phase, (C) different from at the immediate postoperative, (D) just before expiry note the markedly thickened LV walls with a very small LV cavity. (E) Radial strain of the corresponding short-axis view measures 5.408% at immediate postoperative (F) decreasing to 0.050% just before expiry. (G) Hematoxylin and eosin staining of the cardiac xenograft LV walls immediately postoperative from an open biopsy shows a relatively normal myocardium (×400). (H) At autopsy (postoperative day 38), multifocal bacterial colonies with microabscesses (arrows) confirmed the sepsis-involved myocardium (×100). |
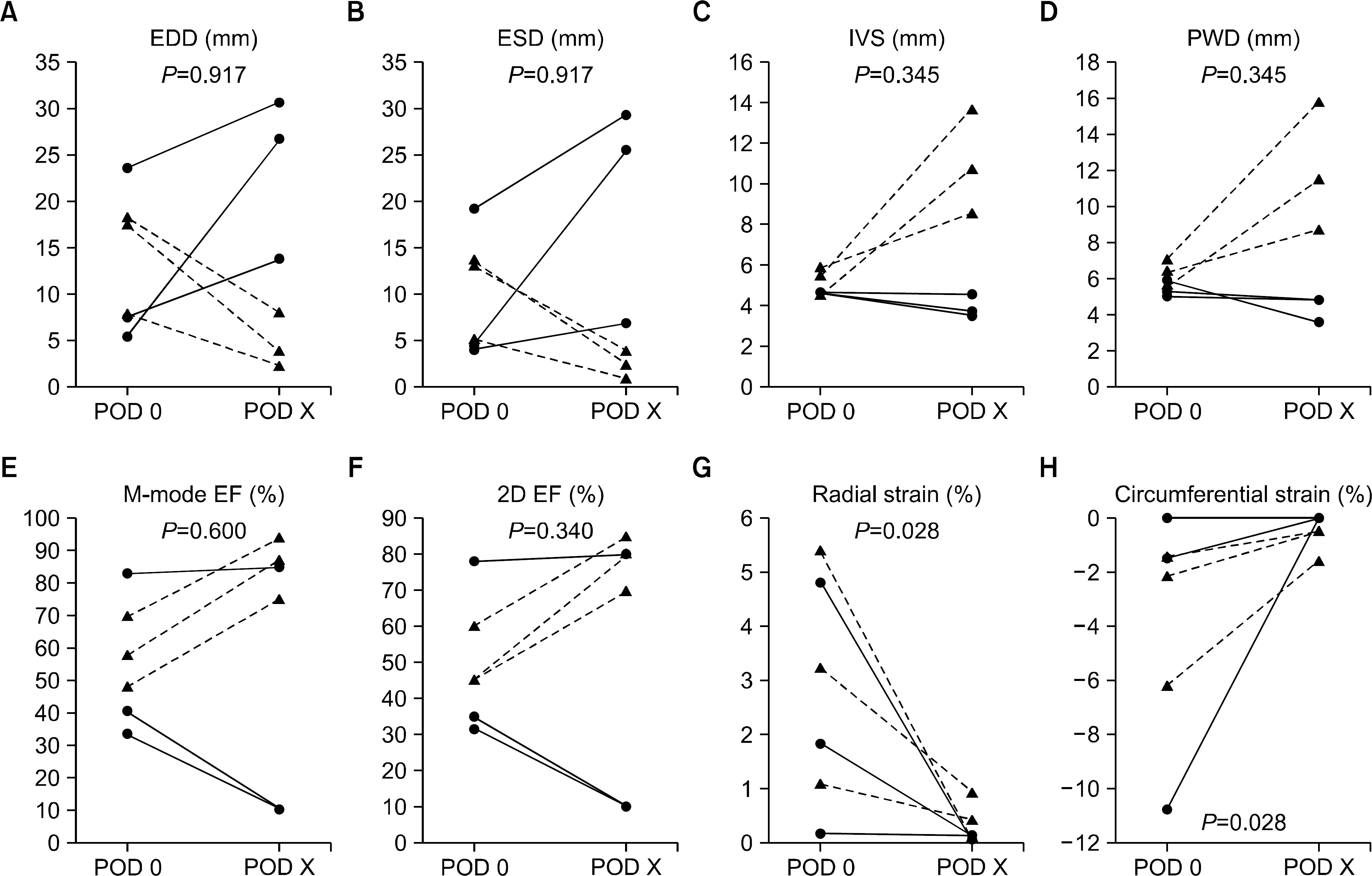 | Fig. 3.In a total of six cases, changes of echocardiographic parameters of the left ventricular (LV) dimension or function between immediate postoperative (postoperative day [POD] 0) and just before expiry (POD X) are compared using a Wilcoxon signed-rank test. Radial and circumferential strain show significant changes (P=0.028). Blue lines with round points represent LV eccentric dilatation; red dots with triangular points represents LV concentric thickening. (A) LV end-diastolic cavity dimension (EDD), (B) LV end-systolic cavity dimension (ESD), (C) interventricular septum (IVS), (D) LV posterior wall dimension (PWD), (E) M-mode LV ejection fraction by Teichholz's method (M-mode EF), (F) two-dimensional LV ejection fraction by Simpson's method (2D-EF), (G) radial strain, and (H) circumferential strain. |
Table 1.
Six cases of xenotransplantationl recipient survival and cardiac xenograft functional parameters
Abbreviations: LV, left ventricular; EDD, LV end-diastolic cavity dimension; ESD, LV end-systolic cavity dimension; Wall, LV wall thickness; M-EF, M-mode LV ejection fractionby Teichholz's method; ZD-EF, two-dimensional LV ejection fraction by Simpson's method; RS, peak systolic radial strain; CS, peak systolic Circumferential strain; ISHLT, InternationalSociety of Heart and Lung Transplantation; AMR, antibody mediated rejection.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download